J Periodontal Implant Sci.
2013 Jun;43(3):111-120. 10.5051/jpis.2013.43.3.111.
Epigenetic biomarkers: a step forward for understanding periodontitis
- Affiliations
-
- 1Division of Epigenomics and Cancer Risk Factors, German Cancer Research Center (DKFZ), Heidelberg, Germany.
- 2Department of Nutritional Science and Food Management, College of Health Science, Ewha Womans University, Seoul, Korea. park.yoonjung@ewha.ac.kr
- KMID: 1798484
- DOI: http://doi.org/10.5051/jpis.2013.43.3.111
Abstract
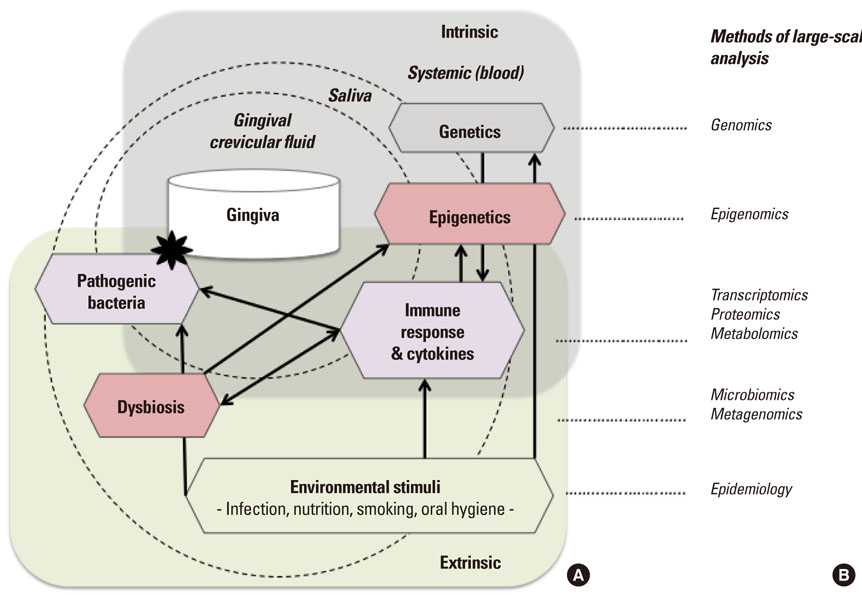
- Periodontitis is a common oral disease that is characterized by infection and inflammation of the tooth supporting tissues. While its incidence is highly associated with outgrowth of the pathogenic microbiome, some patients show signs of predisposition and quickly fall into recurrence after treatment. Recent research using genetic associations of candidates as well as genome-wide analysis highlights that variations in genes related to the inflammatory response are associated with an increased risk of periodontitis. Intriguingly, some of the genes are regulated by epigenetic modifications, supposedly established and reprogrammed in response to environmental stimuli. In addition, the treatment with epigenetic drugs improves treatment of periodontitis in a mouse model. In this review, we highlight some of the recent progress identifying genetic factors associated with periodontitis and point to promising approaches in epigenetic research that may contribute to the understanding of molecular mechanisms involving different responses in individuals and the early detection of predispositions that may guide in future oral treatment and disease prevention.
MeSH Terms
Figure
Cited by 1 articles
-
Epigenetics: general characteristics and implications for oral health
Ji-Yun Seo, Yoon-Jung Park, Young-Ah Yi, Ji-Yun Hwang, In-Bog Lee, Byeong-Hoon Cho, Ho-Hyun Son, Deog-Gyu Seo
Restor Dent Endod. 2015;40(1):14-22. doi: 10.5395/rde.2015.40.1.14.
Reference
-
1. Petersen PE, Ogawa H. The global burden of periodontal disease: towards integration with chronic disease prevention and control. Periodontol 2000. 2012; 60:15–39.
Article2. Petersen PE, Ogawa H. Strengthening the prevention of periodontal disease: the WHO approach. J Periodontol. 2005; 76:2187–2193.
Article3. Genco RJ, Van Dyke TE. Prevention: reducing the risk of CVD in patients with periodontitis. Nat Rev Cardiol. 2010; 7:479–480.
Article4. Tonetti MS. Periodontitis and risk for atherosclerosis: an update on intervention trials. J Clin Periodontol. 2009; 36:Suppl 10. 15–19.
Article5. Lalla E, Papapanou PN. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011; 7:738–748.
Article6. Nibali L, Tatarakis N, Needleman I, Tu YK, D'Aiuto F, Rizzo M, et al. Clinical review: Association between metabolic syndrome and periodontitis: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013; 98:913–920.
Article7. Han DH, Lim SY, Sun BC, Paek D, Kim HD. The association of metabolic syndrome with periodontal disease is confounded by age and smoking in a Korean population: the Shiwha-Banwol Environmental Health Study. J Clin Periodontol. 2010; 37:609–616.
Article8. Kwon YE, Ha JE, Paik DI, Jin BH, Bae KH. The relationship between periodontitis and metabolic syndrome among a Korean nationally representative sample of adults. J Clin Periodontol. 2011; 38:781–786.
Article9. Awano S, Ansai T, Takata Y, Soh I, Akifusa S, Hamasaki T, et al. Oral health and mortality risk from pneumonia in the elderly. J Dent Res. 2008; 87:334–339.
Article10. Paju S, Scannapieco FA. Oral biofilms, periodontitis, and pulmonary infections. Oral Dis. 2007; 13:508–512.
Article11. Lundberg K, Wegner N, Yucel-Lindberg T, Venables PJ. Periodontitis in RA-the citrullinated enolase connection. Nat Rev Rheumatol. 2010; 6:727–730.
Article12. Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005; 38:135–187.
Article13. Hajishengallis G, Lambris JD. Complement and dysbiosis in periodontal disease. Immunobiology. 2012; 217:1111–1116.
Article14. Darveau RP, Hajishengallis G, Curtis MA. Porphyromonas gingivalis as a potential community activist for disease. J Dent Res. 2012; 91:816–820.
Article15. Van Dyke TE, Sheilesh D. Risk factors for periodontitis. J Int Acad Periodontol. 2005; 7:3–7.16. Van der Velden U, Abbas F, Armand S, Loos BG, Timmerman MF, Van der Weijden GA, et al. Java project on periodontal diseases. The natural development of periodontitis: risk factors, risk predictors and risk determinants. J Clin Periodontol. 2006; 33:540–548.
Article17. Yamamoto K, Kobayashi T, Grossi S, Ho AW, Genco RJ, Yoshie H, et al. Association of Fcgamma receptor IIa genotype with chronic periodontitis in Caucasians. J Periodontol. 2004; 75:517–522.
Article18. Fu Y, Korostoff JM, Fine DH, Wilson ME. Fc gamma receptor genes as risk markers for localized aggressive periodontitis in African-Americans. J Periodontol. 2002; 73:517–523.
Article19. Yoshihara A, Sugita N, Yamamoto K, Kobayashi T, Miyazaki H, Yoshi H. Analysis of vitamin D and Fcgamma receptor polymorphisms in Japanese patients with generalized early-onset periodontitis. J Dent Res. 2001; 80:2051–2054.
Article20. Chai L, Song YQ, Zee KY, Leung WK. SNPs of Fc-gamma receptor genes and chronic periodontitis. J Dent Res. 2010; 89:705–710.
Article21. Maria de Freitas N, Imbronito AV, Neves AC, Nunes FD, Pustiglioni FE, Lotufo RF. Analysis of IL-1A(-889) and TNFA(-308) gene polymorphism in Brazilian patients with generalized aggressive periodontitis. Eur Cytokine Netw. 2007; 18:142–147.22. Komatsu Y, Galicia JC, Kobayashi T, Yamazaki K, Yoshie H. Association of interleukin-1 receptor antagonist +2018 gene polymorphism with Japanese chronic periodontitis patients using a novel genotyping method. Int J Immunogenet. 2008; 35:165–170.
Article23. Kang BY, Choi YK, Choi WH, Kim KT, Choi SS, Kim K, et al. Two polymorphisms of interleukin-4 gene in Korean adult periodontitis. Arch Pharm Res. 2003; 26:482–486.
Article24. Michel J, Gonzales JR, Wunderlich D, Diete A, Herrmann JM, Meyle J. Interleukin-4 polymorphisms in early onset periodontitis. J Clin Periodontol. 2001; 28:483–488.
Article25. Aoyagi T, Sugawara-Aoyagi M, Yamazaki K, Hara K. Interleukin 4 (IL-4) and IL-6-producing memory T-cells in peripheral blood and gingival tissue in periodontitis patients with high serum antibody titers to Porphyromonas gingivalis. Oral Microbiol Immunol. 1995; 10:304–310.
Article26. Stefani FA, Viana MB, Dupim AC, Brito JA, Gomez RS, da Costa JE, et al. Expression, polymorphism and methylation pattern of interleukin-6 in periodontal tissues. Immunobiology. 2013; 218:1012–1017.
Article27. Mellati E, Arab HR, Tavakkol-Afshari J, Ebadian AR, Radvar M. Analysis of -1082 IL-10 gene polymorphism in Iranian patients with generalized aggressive periodontitis. Med Sci Monit. 2007; 13:CR510–CR514.28. Babel N, Cherepnev G, Babel D, Tropmann A, Hammer M, Volk HD, et al. Analysis of tumor necrosis factor-alpha, transforming growth factor-beta, interleukin-10, IL-6, and interferon-gamma gene polymorphisms in patients with chronic periodontitis. J Periodontol. 2006; 77:1978–1983.
Article29. Noack B, Gorgens H, Lorenz K, Ziegler A, Hoffmann T, Schackert HK. TLR4 and IL-18 gene variants in aggressive periodontitis. J Clin Periodontol. 2008; 35:1020–1026.30. Noack B, Gorgens H, Lorenz K, Schackert HK, Hoffmann T. TLR4 and IL-18 gene variants in chronic periodontitis: impact on disease susceptibility and severity. Immunol Invest. 2009; 38:297–310.
Article31. Fassmann A, Holla LI, Buckova D, Vasku A, Znojil V, Vanek J. Polymorphisms in the +252(A/G) lymphotoxin-alpha and the -308(A/G) tumor necrosis factor-alpha genes and susceptibility to chronic periodontitis in a Czech population. J Periodontal Res. 2003; 38:394–399.
Article32. Garlet GP, Trombone AP, Menezes R, Letra A, Repeke CE, Vieira AE, et al. The use of chronic gingivitis as reference status increases the power and odds of periodontitis genetic studies: a proposal based in the exposure concept and clearer resistance and susceptibility phenotypes definition. J Clin Periodontol. 2012; 39:323–332.
Article33. Deng H, Liu F, Pan Y, Jin X, Wang H, Cao J. BsmI, TaqI, ApaI, and FokI polymorphisms in the vitamin D receptor gene and periodontitis: a meta-analysis of 15 studies including 1338 cases and 1302 controls. J Clin Periodontol. 2011; 38:199–207.
Article34. Park KS, Nam JH, Choi J. The short vitamin D receptor is associated with increased risk for generalized aggressive periodontitis. J Clin Periodontol. 2006; 33:524–528.
Article35. Sun JL, Meng HX, Cao CF, Tachi Y, Shinohara M, Ueda M, et al. Relationship between vitamin D receptor gene polymorphism and periodontitis. J Periodontal Res. 2002; 37:263–267.
Article36. Nicu EA, Laine ML, Morre SA, Van der Velden U, Loos BG. Soluble CD14 in periodontitis. Innate Immun. 2009; 15:121–128.
Article37. Laine ML, Morre SA, Murillo LS, van Winkelhoff AJ, Peña AS. CD14 and TLR4 gene polymorphisms in adult periodontitis. J Dent Res. 2005; 84:1042–1046.
Article38. James JA, Poulton KV, Haworth SE, Payne D, McKay IJ, Clarke FM, et al. Polymorphisms of TLR4 but not CD14 are associated with a decreased risk of aggressive periodontitis. J Clin Periodontol. 2007; 34:111–117.
Article39. Holla LI, Jurajda M, Fassmann A, Dvorakova N, Znojil V, Vacha J. Genetic variations in the matrix metalloproteinase-1 promoter and risk of susceptibility and/or severity of chronic periodontitis in the Czech population. J Clin Periodontol. 2004; 31:685–690.
Article40. Li D, Cai Q, Ma L, Wang M, Ma J, Zhang W, et al. Association between MMP-1 g.-1607dupG polymorphism and periodontitis susceptibility: a meta-analysis. PLoS One. 2013; 8:e59513.
Article41. Emingil G, Berdeli A, Baylas H, Saygan BH, Gürkan A, Köse T, et al. Toll-like receptor 2 and 4 gene polymorphisms in generalized aggressive periodontitis. J Periodontol. 2007; 78:1968–1977.
Article42. Berdeli A, Emingil G, Han Saygan B, Gurkan A, Atilla G, Köse T, et al. TLR2 Arg753Gly, TLR4 Asp299Gly and Thr399Ile gene polymorphisms are not associated with chronic periodontitis in a Turkish population. J Clin Periodontol. 2007; 34:551–557.
Article43. Daing A, Singh SV, Saimbi CS, Khan MA, Rath SK. Cyclooxygenase 2 gene polymorphisms and chronic periodontitis in a North Indian population: a pilot study. J Periodontal Implant Sci. 2012; 42:151–157.
Article44. Li Y, Xu L, Hasturk H, Kantarci A, DePalma SR, Van Dyke TE. Localized aggressive periodontitis is linked to human chromosome 1q25. Hum Genet. 2004; 114:291–297.
Article45. Chai L, Song YQ, Leung WK. Genetic polymorphism studies in periodontitis and Fcγ receptors. J Periodontal Res. 2012; 47:273–285.
Article46. Laine ML, Crielaard W, Loos BG. Genetic susceptibility to periodontitis. Periodontol 2000. 2012; 58:37–68.
Article47. Grant MM. What do 'omic technologies have to offer periodontal clinical practice in the future? J Periodontal Res. 2012; 47:2–14.
Article48. Schaefer AS, Richter GM, Nothnagel M, Manke T, Dommisch H, Jacobs G, et al. A genome-wide association study identifies GLT6D1 as a susceptibility locus for periodontitis. Hum Mol Genet. 2010; 19:553–562.
Article49. Divaris K, Monda KL, North KE, Olshan AF, Reynolds LM, Hsueh WC, et al. Exploring the genetic basis of chronic periodontitis: a genome-wide association study. Hum Mol Genet. 2013; 22:2312–2324.
Article50. Demmer RT, Behle JH, Wolf DL, Handfield M, Kebschull M, Celenti R, et al. Transcriptomes in healthy and diseased gingival tissues. J Periodontol. 2008; 79:2112–2124.
Article51. Papapanou PN, Behle JH, Kebschull M, Celenti R, Wolf DL, Handfield M, et al. Subgingival bacterial colonization profiles correlate with gingival tissue gene expression. BMC Microbiol. 2009; 9:221.
Article52. Papapanou PN, Sedaghatfar MH, Demmer RT, Wolf DL, Yang J, Roth GA, et al. Periodontal therapy alters gene expression of peripheral blood monocytes. J Clin Periodontol. 2007; 34:736–747.
Article53. Kojima T, Andersen E, Sanchez JC, Wilkins MR, Hochstrasser DF, Pralong WF, et al. Human gingival crevicular fluid contains MRP8 (S100A8) and MRP14 (S100A9), two calcium-binding proteins of the S100 family. J Dent Res. 2000; 79:740–747.
Article54. Wu Y, Shu R, Luo LJ, Ge LH, Xie YF. Initial comparison of proteomic profiles of whole unstimulated saliva obtained from generalized aggressive periodontitis patients and healthy control subjects. J Periodontal Res. 2009; 44:636–644.
Article55. Barnes VM, Teles R, Trivedi HM, Devizio W, Xu T, Mitchell MW, et al. Acceleration of purine degradation by periodontal diseases. J Dent Res. 2009; 88:851–855.
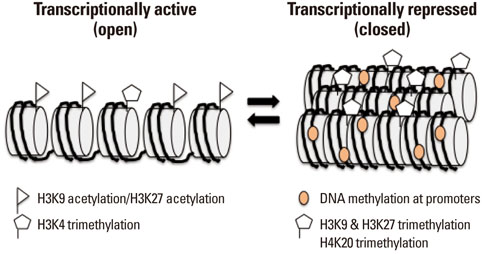
Article56. Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011; 21:381–395.
Article57. Wei G, Hu G, Cui K, Zhao K. Genome-wide mapping of nucleosome occupancy, histone modifications, and gene expression using next-generation sequencing technology. Methods Enzymol. 2012; 513:297–313.
Article58. Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007; 129:823–837.
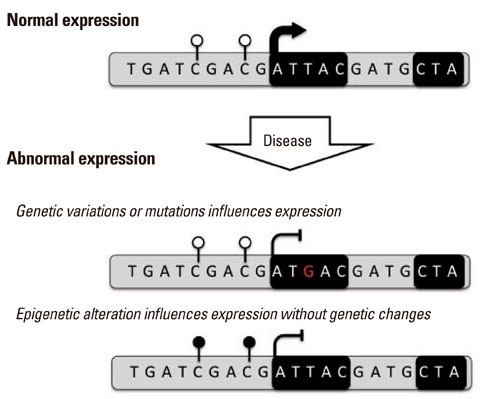
Article59. Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012; 13:484–492.
Article60. Tan L, Shi YG. Tet family proteins and 5-hydroxymethylcytosine in development and disease. Development. 2012; 139:1895–1902.
Article61. Gal-Yam EN, Egger G, Iniguez L, Holster H, Einarsson S, Zhang X, et al. Frequent switching of Polycomb repressive marks and DNA hypermethylation in the PC3 prostate cancer cell line. Proc Natl Acad Sci U S A. 2008; 105:12979–12984.
Article62. Lindroth AM, Park YJ, McLean CM, Dokshin GA, Persson JM, Herman H, et al. Antagonism between DNA and H3K27 methylation at the imprinted Rasgrf1 locus. PLoS Genet. 2008; 4:e1000145.
Article63. Cortessis VK, Thomas DC, Levine AJ, Breton CV, Mack TM, Siegmund KD, et al. Environmental epigenetics: prospects for studying epigenetic mediation of exposure-response relationships. Hum Genet. 2012; 131:1565–1589.
Article64. Kilpinen H, Dermitzakis ET. Genetic and epigenetic contribution to complex traits. Hum Mol Genet. 2012; 21:R24–R28.
Article65. Bonasio R, Tu S, Reinberg D. Molecular signals of epigenetic states. Science. 2010; 330:612–616.
Article66. Tsai PC, Spector TD, Bell JT. Using epigenome-wide association scans of DNA methylation in age-related complex human traits. Epigenomics. 2012; 4:511–526.
Article67. Li H, Deng H. Systems genetics, bioinformatics and eQTL mapping. Genetica. 2010; 138:915–924.
Article68. Baranzini SE, Mudge J, van Velkinburgh JC, Khankhanian P, Khrebtukova I, Miller NA, et al. Genome, epigenome and RNA sequences of monozygotic twins discordant for multiple sclerosis. Nature. 2010; 464:1351–1356.
Article69. Williams SC. Genetics: searching for answers. Nature. 2012; 491:S4–S6.
Article70. How Kit A, Nielsen HM, Tost J. DNA methylation based biomarkers: practical considerations and applications. Biochimie. 2012; 94:2314–2337.
Article71. Mendenhall EM, Bernstein BE. Chromatin state maps: new technologies, new insights. Curr Opin Genet Dev. 2008; 18:109–115.
Article72. Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat Rev Genet. 2013; 14:204–220.
Article73. Meissner A. Epigenetic modifications in pluripotent and differentiated cells. Nat Biotechnol. 2010; 28:1079–1088.
Article74. McDonald OG, Wu H, Timp W, Doi A, Feinberg AP. Genome-scale epigenetic reprogramming during epithelial-to-mesenchymal transition. Nat Struct Mol Biol. 2011; 18:867–874.
Article75. Kellermayer R. Epigenetics and the developmental origins of inflammatory bowel diseases. Can J Gastroenterol. 2012; 26:909–915.
Article76. Barros SP, Offenbacher S. Epigenetics: connecting environment and genotype to phenotype and disease. J Dent Res. 2009; 88:400–408.
Article77. Alegria-Torres JA, Baccarelli A, Bollati V. Epigenetics and lifestyle. Epigenomics. 2011; 3:267–277.
Article78. Wen H, Schaller MA, Dou Y, Hogaboam CM, Kunkel SL. Dendritic cells at the interface of innate and acquired immunity: the role for epigenetic changes. J Leukoc Biol. 2008; 83:439–446.
Article79. Fitzpatrick DR, Wilson CB. Methylation and demethylation in the regulation of genes, cells, and responses in the immune system. Clin Immunol. 2003; 109:37–45.
Article80. O'Shea JJ, Lahesmaa R, Vahedi G, Laurence A, Kanno Y. Genomic views of STAT function in CD4+ T helper cell differentiation. Nat Rev Immunol. 2011; 11:239–250.81. Nielsen HM, Tost J. Epigenetic changes in inflammatory and autoimmune diseases. Subcell Biochem. 2012; 61:455–478.
Article82. Villagra A, Sotomayor EM, Seto E. Histone deacetylases and the immunological network: implications in cancer and inflammation. Oncogene. 2010; 29:157–173.
Article83. Barnes PJ. Targeting the epigenome in the treatment of asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009; 6:693–696.
Article84. Sullivan KE, Reddy AB, Dietzmann K, Suriano AR, Kocieda VP, Stewart M, et al. Epigenetic regulation of tumor necrosis factor alpha. Mol Cell Biol. 2007; 27:5147–5160.
Article85. White GP, Watt PM, Holt BJ, Holt PG. Differential patterns of methylation of the IFN-gamma promoter at CpG and non-CpG sites underlie differences in IFN-gamma gene expression between human neonatal and adult CD45RO- T cells. J Immunol. 2002; 168:2820–2827.
Article86. Adcock IM, Tsaprouni L, Bhavsar P, Ito K. Epigenetic regulation of airway inflammation. Curr Opin Immunol. 2007; 19:694–700.
Article87. Shuto T, Furuta T, Oba M, Xu H, Li JD, Cheung J, et al. Promoter hypomethylation of Toll-like receptor-2 gene is associated with increased proinflammatory response toward bacterial peptidoglycan in cystic fibrosis bronchial epithelial cells. FASEB J. 2006; 20:782–784.
Article88. Takahashi K, Sugi Y, Hosono A, Kaminogawa S. Epigenetic regulation of TLR4 gene expression in intestinal epithelial cells for the maintenance of intestinal homeostasis. J Immunol. 2009; 183:6522–6529.
Article89. Kanzler H, Barrat FJ, Hessel EM, Coffman RL. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med. 2007; 13:552–559.
Article90. Hodge DR, Xiao W, Clausen PA, Heidecker G, Szyf M, Farrar WL. Interleukin-6 regulation of the human DNA methyltransferase (HDNMT) gene in human erythroleukemia cells. J Biol Chem. 2001; 276:39508–39511.
Article91. Hmadcha A, Bedoya FJ, Sobrino F, Pintado E. Methylation-dependent gene silencing induced by interleukin 1beta via nitric oxide production. J Exp Med. 1999; 190:1595–1604.
Article92. Bayarsaihan D. Epigenetic mechanisms in inflammation. J Dent Res. 2011; 90:9–17.
Article93. Zhang S, Crivello A, Offenbacher S, Moretti A, Paquette DW, Barros SP. Interferon-gamma promoter hypomethylation and increased expression in chronic periodontitis. J Clin Periodontol. 2010; 37:953–961.
Article94. Zhang S, Barros SP, Moretti AJ, Yu N, Zhou J, Preisser JS, et al. Epigenetic regulation of TNFA expression in periodontal disease. J Periodontol. 2013; 01. 31. [Epub]. http://dx.doi.org/10.1902/jop.2013.120294.95. Gorska R, Gregorek H, Kowalski J, Laskus-Perendyk A, Syczewska M, Madalinski K. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J Clin Periodontol. 2003; 30:1046–1052.
Article96. Salvi GE, Brown CE, Fujihashi K, Kiyono H, Smith FW, Beck JD, et al. Inflammatory mediators of the terminal dentition in adult and early onset periodontitis. J Periodontal Res. 1998; 33:212–225.
Article97. Pinho Mde N, Pereira LB, de Souza SL, Palioto DB, Grisi MF, Novaes AB Jr, et al. Short-term effect of COX-2 selective inhibitor as an adjunct for the treatment of periodontal disease: a clinical double-blind study in humans. Braz Dent J. 2008; 19:323–328.
Article98. Zhang S, Barros SP, Niculescu MD, Moretti AJ, Preisser JS, Offenbacher S. Alteration of PTGS2 promoter methylation in chronic periodontitis. J Dent Res. 2010; 89:133–137.
Article99. Loo WT, Jin L, Cheung MN, Wang M, Chow LW. Epigenetic change in E-cadherin and COX-2 to predict chronic periodontitis. J Transl Med. 2010; 8:110.100. Cantley MD, Bartold PM, Marino V, Fairlie DP, Le GT, Lucke AJ, et al. Histone deacetylase inhibitors and periodontal bone loss. J Periodontal Res. 2011; 46:697–703.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Genetic and epigenetic alterations of colorectal cancer
- Pathogenesis and biomarkers of colorectal cancer by epigenetic alteration
- Looking Forward to Saying Goodbye to COVID-19 and a Step Forward
- Analysis of Periodontitis Biomarker Expression in Gingival Crevicular Fluids
- Potential Epigenetic Biomarkers for Prostate Cancer Screening