Yonsei Med J.
2013 Sep;54(5):1158-1167. 10.3349/ymj.2013.54.5.1158.
NAD(P)H: Quinone Oxidoreductase 1 and NRH:Quinone Oxidoreductase 2 Polymorphisms in Papillary Thyroid Microcarcinoma: Correlation with Phenotype
- Affiliations
-
- 1Department of Pathology, Daejeon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Daejeon, Korea.
- 2Research Center for Endocrine and Metabolic Diseases, Chungnam National University Hospital, Daejeon, Korea. ysmrj@cnuh.co.kr
- 3College of Biological Sciences and Biotechnology, Department of Bioscience, Chungnam National University, Daejeon, Korea.
- 4Cheong Shim International Academy, Gapyeong, Korea.
- 5Department of Internal Medicine, Chungnam National University School of Medicine, Daejeon, Korea.
- KMID: 1793163
- DOI: http://doi.org/10.3349/ymj.2013.54.5.1158
Abstract
- PURPOSE
NAD(P)H:Quinone Oxidoreductase 1 (NQO1) C609T missense variant (NQO1*2) and 29 basepair (bp)-insertion/deletion (I29/D) polymorphism of the NRH:Quinone Oxidoreductase 2 (NQO2) gene promoter have been proposed as predictive and prognostic factors for cancer development and progression. The purpose of this study is to investigate the relationship between NQO1/NQO2 genotype and clinico-pathological features of papillary thyroid microcarcinoma (PTMC).
MATERIALS AND METHODS
Genomic DNA was isolated from 243 patients; and clinical data were retrospectively analyzed. NQO1*2 and tri-allelic polymorphism of NQO2 were investigated by polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis.
RESULTS
PTMC with NQO1*2 frequently exhibited extra-thyroidal extension as compared to PTMC with wild-type NQO1 (p=0.039). There was a significant relationship between I29/I29 homozygosity of NQO2 and lymph node metastasis (p=0.042). Multivariate analysis showed that the I29/I29 genotype was associated with an increased risk of lymph node metastasis (OR, 2.24; 95% CI, 1.10-4.56; p=0.026).
CONCLUSION
NQO1*2 and I29 allele of the NQO2 are associated with aggressive clinical phenotypes of PTMC, and the I29 allele represents a putative prognostic marker for PTMC.
MeSH Terms
-
Adult
Carcinoma, Papillary/*genetics/pathology
DNA Mutational Analysis
Female
Genetic Predisposition to Disease
Humans
Immunohistochemistry
Male
Middle Aged
Multivariate Analysis
Mutagenesis, Insertional
Mutation, Missense
NAD(P)H Dehydrogenase (Quinone)/chemistry/*genetics
Phenotype
Polymorphism, Genetic
Prognosis
Promoter Regions, Genetic
Retrospective Studies
Sequence Analysis, Protein
Sequence Deletion
Thyroid Neoplasms/*genetics/pathology
NAD(P)H Dehydrogenase (Quinone)
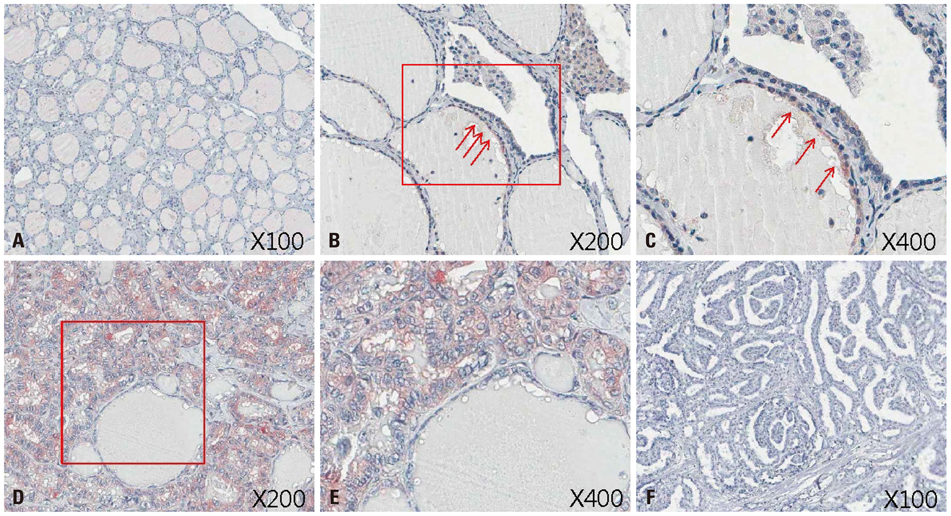
Figure
Reference
-
1. Shaw PM, Reiss A, Adesnik M, Nebert DW, Schembri J, Jaiswal AK. The human dioxin-inducible NAD(P)H: quinone oxidoreductase cDNA-encoded protein expressed in COS-1 cells is identical to diaphorase 4. Eur J Biochem. 1991; 195:171–176.
Article2. Jaiswal AK, Burnett P, Adesnik M, McBride OW. Nucleotide and deduced amino acid sequence of a human cDNA (NQO2) corresponding to a second member of the NAD(P)H:quinone oxidoreductase gene family. Extensive polymorphism at the NQO2 gene locus on chromosome 6. Biochemistry. 1990; 29:1899–1906.
Article3. Long DJ 2nd, Jaiswal AK. Mouse NRH:quinone oxidoreductase (NQO2): cloning of cDNA and gene- and tissue-specific expression. Gene. 2000; 252:107–117.
Article4. Iskander K, Gaikwad A, Paquet M, Long DJ 2nd, Brayton C, Barrios R, et al. Lower induction of p53 and decreased apoptosis in NQO1-null mice lead to increased sensitivity to chemical-induced skin carcinogenesis. Cancer Res. 2005; 65:2054–2058.
Article5. Iskander K, Barrios RJ, Jaiswal AK. NRH:quinone oxidoreductase 2-deficient mice are highly susceptible to radiation-induced B-cell lymphomas. Clin Cancer Res. 2009; 15:1534–1542.
Article6. Ross D, Beall H, Traver RD, Siegel D, Phillips RM, Gibson NW. Bioactivation of quinones by DT-diaphorase, molecular, biochemical, and chemical studies. Oncol Res. 1994; 6:493–500.7. Siegel D, Anwar A, Winski SL, Kepa JK, Zolman KL, Ross D. Rapid polyubiquitination and proteasomal degradation of a mutant form of NAD(P)H:quinone oxidoreductase 1. Mol Pharmacol. 2001; 59:263–268.
Article8. Rothman N, Smith MT, Hayes RB, Traver RD, Hoener B, Campleman S, et al. Benzene poisoning, a risk factor for hematological malignancy, is associated with the NQO1 609C-->T mutation and rapid fractional excretion of chlorzoxazone. Cancer Res. 1997; 57:2839–2842.9. Fagerholm R, Hofstetter B, Tommiska J, Aaltonen K, Vrtel R, Syrjäkoski K, et al. NAD(P)H:quinone oxidoreductase 1 NQO1*2 genotype (P187S) is a strong prognostic and predictive factor in breast cancer. Nat Genet. 2008; 40:844–853.
Article10. Wang W, Le WD, Pan T, Stringer JL, Jaiswal AK. Association of NRH:quinone oxidoreductase 2 gene promoter polymorphism with higher gene expression and increased susceptibility to Parkinson's disease. J Gerontol A Biol Sci Med Sci. 2008; 63:127–134.
Article11. Wang W, Jaiswal AK. Sp3 repression of polymorphic human NRH:quinone oxidoreductase 2 gene promoter. Free Radic Biol Med. 2004; 37:1231–1243.
Article12. Yu KD, Di GH, Yuan WT, Fan L, Wu J, Hu Z, et al. Functional polymorphisms, altered gene expression and genetic association link NRH:quinone oxidoreductase 2 to breast cancer with wild-type p53. Hum Mol Genet. 2009; 18:2502–2517.
Article13. Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988-2005. Cancer. 2009; 115:3801–3807.
Article14. Kazakov VS, Demidchik EP, Astakhova LN. Thyroid cancer after Chernobyl. Nature. 1992; 359:21.
Article15. Chen W, Man N, Shan Z, Teng W. Effects of long-term exposure to iodine excess on the apoptosis of thyrocytes in Wistar rats. Exp Clin Endocrinol Diabetes. 2011; 119:1–8.
Article16. Gossen JA, de Leeuw WJ, Molijn AC, Vijg J. Plasmid rescue from transgenic mouse DNA using LacI repressor protein conjugated to magnetic beads. Biotechniques. 1993; 14:624–629.17. Kouniavsky G, Zeiger MA. Thyroid tumorigenesis and molecular markers in thyroid cancer. Curr Opin Oncol. 2010; 22:23–29.
Article18. Lafuente MJ, Casterad X, Trias M, Ascaso C, Molina R, Ballesta A, et al. NAD(P)H:quinone oxidoreductase-dependent risk for colorectal cancer and its association with the presence of K-ras mutations in tumors. Carcinogenesis. 2000; 21:1813–1819.
Article19. Farid NR. P53 mutations in thyroid carcinoma: tidings from an old foe. J Endocrinol Invest. 2001; 24:536–545.
Article20. Jo YS, Huang S, Kim YJ, Lee IS, Kim SS, Kim JR, et al. Diagnostic value of pyrosequencing for the BRAF V600E mutation in ultrasound-guided fine-needle aspiration biopsy samples of thyroid incidentalomas. Clin Endocrinol (Oxf). 2009; 70:139–144.
Article21. Kelsey KT, Ross D, Traver RD, Christiani DC, Zuo ZF, Spitz MR, et al. Ethnic variation in the prevalence of a common NAD(P)H quinone oxidoreductase polymorphism and its implications for anti-cancer chemotherapy. Br J Cancer. 1997; 76:852–854.
Article22. Yu KD, Di GH, Fan L, Hu Z, Chen AX, Shao ZM. Caution regarding genotyping methodology for a tri-allelic polymorphism in the novel breast cancer susceptibility gene NQO2. Breast Cancer Res Treat. 2009; 118:647–649.
Article23. Belinsky M, Jaiswal AK. NAD(P)H:quinone oxidoreductase1 (DT-diaphorase) expression in normal and tumor tissues. Cancer Metastasis Rev. 1993; 12:103–117.
Article24. Chen XL, Kunsch C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr Pharm Des. 2004; 10:879–891.
Article25. Chao C, Zhang ZF, Berthiller J, Boffetta P, Hashibe M. NAD(P)H:quinone oxidoreductase 1 (NQO1) Pro187Ser polymorphism and the risk of lung, bladder, and colorectal cancers: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006; 15:979–987.
Article26. Long DJ 2nd, Gaikwad A, Multani A, Pathak S, Montgomery CA, Gonzalez FJ, et al. Disruption of the NAD(P)H:quinone oxidoreductase 1 (NQO1) gene in mice causes myelogenous hyperplasia. Cancer Res. 2002; 62:3030–3036.27. Maier J, van Steeg H, van Oostrom C, Karger S, Paschke R, Krohn K. Deoxyribonucleic acid damage and spontaneous mutagenesis in the thyroid gland of rats and mice. Endocrinology. 2006; 147:3391–3397.
Article28. Krause K, Karger S, Schierhorn A, Poncin S, Many MC, Fuhrer D. Proteomic profiling of cold thyroid nodules. Endocrinology. 2007; 148:1754–1763.
Article29. Krohn K, Maier J, Paschke R. Mechanisms of disease: hydrogen peroxide, DNA damage and mutagenesis in the development of thyroid tumors. Nat Clin Pract Endocrinol Metab. 2007; 3:713–720.
Article30. Siegel D, Ross D. Immunodetection of NAD(P)H:quinone oxidoreductase 1 (NQO1) in human tissues. Free Radic Biol Med. 2000; 29:246–253.31. Yan F, Yang WK, Li XY, Lin TT, Lun YN, Lin F, et al. A trifunctional enzyme with glutathione S-transferase, glutathione peroxidase and superoxide dismutase activity. Biochim Biophys Acta. 2008; 1780:869–872.
Article32. Saavedra HI, Knauf JA, Shirokawa JM, Wang J, Ouyang B, Elisei R, et al. The RAS oncogene induces genomic instability in thyroid PCCL3 cells via the MAPK pathway. Oncogene. 2000; 19:3948–3954.
Article33. Song SY, Jeong SY, Park HJ, Park SI, Kim DK, Kim YH, et al. Clinical significance of NQO1 C609T polymorphisms after postoperative radiation therapy in completely resected non-small cell lung cancer. Lung Cancer. 2010; 68:278–282.
Article34. Shen J, Barrios RJ, Jaiswal AK. Inactivation of the quinone oxidoreductases NQO1 and NQO2 strongly elevates the incidence and multiplicity of chemically induced skin tumors. Cancer Res. 2010; 70:1006–1014.
Article35. Francisco DC, Peddi P, Hair JM, Flood BA, Cecil AM, Kalogerinis PT, et al. Induction and processing of complex DNA damage in human breast cancer cells MCF-7 and nonmalignant MCF-10A cells. Free Radic Biol Med. 2008; 44:558–569.
Article36. Holt SM, Georgakilas AG. Detection of complex DNA damage in gamma-irradiated acute lymphoblastic leukemia Pre-b NALM-6 cells. Radiat Res. 2007; 168:527–534.
Article37. Ziech D, Franco R, Pappa A, Panayiotidis MI. Reactive oxygen species (ROS)--induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res. 2011; 711:167–173.
Article38. Ushijima T. Detection and interpretation of altered methylation patterns in cancer cells. Nat Rev Cancer. 2005; 5:223–231.
Article39. Georgakilas AG, Aziz K, Ziech D, Georgakila S, Panayiotidis MI. BRCA1 involvement in toxicological responses and human cancer etiology. Toxicol Lett. 2009; 188:77–83.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- NAD(P)H-quinone oxidoreductase-1 silencing modulates cytoprotection related protein expression in cisplatin cytotoxicity
- Association between Expression of NAD(P)H: Quinone Oxidoreductase 1 (NQO1) and Synergistic Effect of Ionizing Radiation and beta-lapachone on Human Lung Cancer Cell Line (A549)
- An Association between 609 C --> T Polymorphism in NAD(P)H: Quinone Oxidoreductase 1 (NQO1) Gene and Blood Glucose Levels in Korean Population
- Interactive Effect of Smoking and NQO1 Haplotypes on Lung Cancer Risk
- beta-Lapachone suppresses radiation-induced activation of nuclear factor-kappaB