Yonsei Med J.
2013 Sep;54(5):1149-1157. 10.3349/ymj.2013.54.5.1149.
Sendai F/HN Viroplexes for Efficient Transfection of Leukemic T Cells
- Affiliations
-
- 1Department of Biomedical Laboratory Science, Yonsei University, Wonju, Korea. parkys@yonsei.ac.kr
- 2Department of Biomedical Laboratory Science, Korea Nazarene University, Cheonan, Korea.
- 3Department of Biomedical Laboratory Science, Konyang University, Daejeon, Korea.
- 4Department of Bioscience and Biotechnology, Konkuk University, Seoul, Korea.
- KMID: 1793162
- DOI: http://doi.org/10.3349/ymj.2013.54.5.1149
Abstract
- PURPOSE
Most chemical transfection reagents are ineffective for the transfection of cells in suspension, such as leukemic cell and stem cell lineages. We developed two different types of viroplexes, cationic Sendai F/HN viroplexes (CSVs) and protamine sulfate-condensed cationic Sendai F/HN viroplexes (PCSVs) for the efficient transfection of T-leukemic cells.
MATERIALS AND METHODS
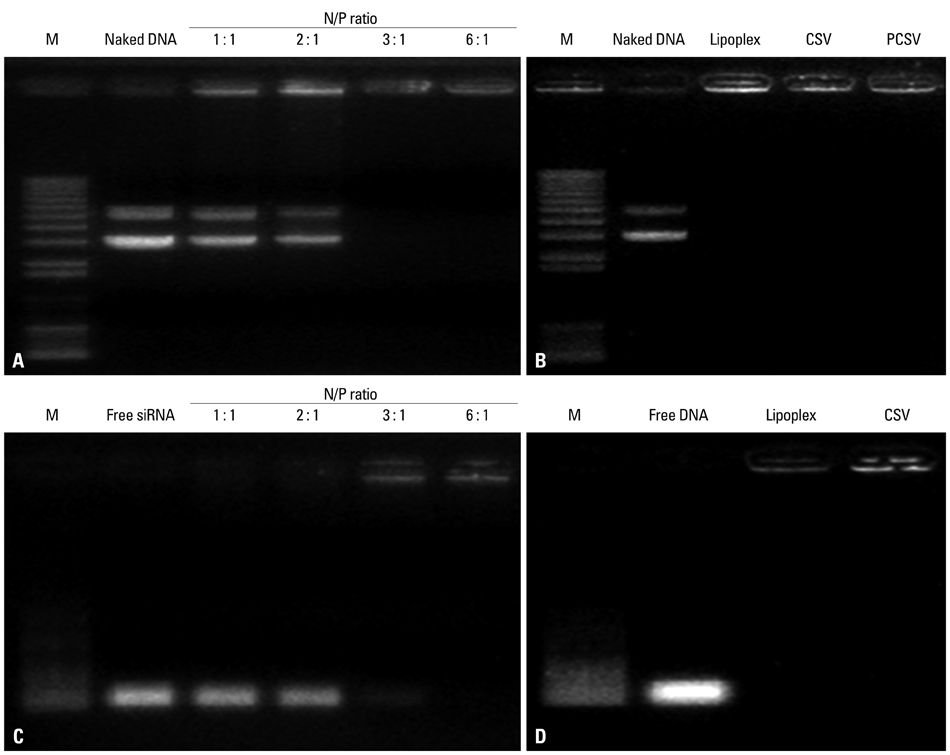
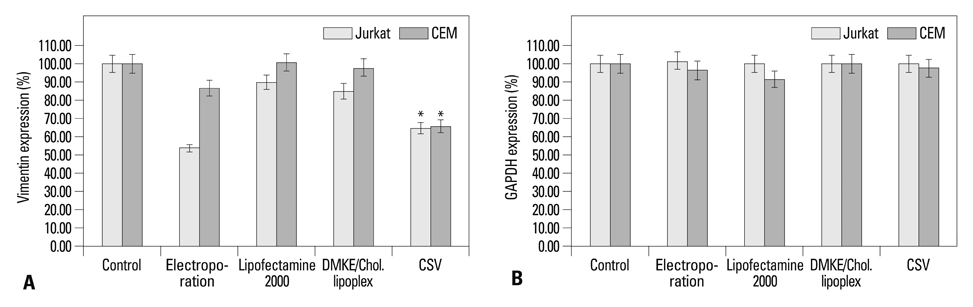
The viroplex systems were prepared by reconstitution of fusogenic Sendai F/HN proteins in DMKE (O,O'-dimyristyl-N-lysyl glutamate) cationic liposomes. The viroplexes were further optimized for plasmid DNA and siRNA delivery to suspension cells. The particle size and surface charge of the viroplexes were analyzed with a zeta-sizer. Transfection of plasmid DNA (pDNA) and small interfering RNA (siRNA) by CSVs or PCSV was evaluated by measurement of transgene expression, confocal microscopy, FACS, and RT-PCR.
RESULTS
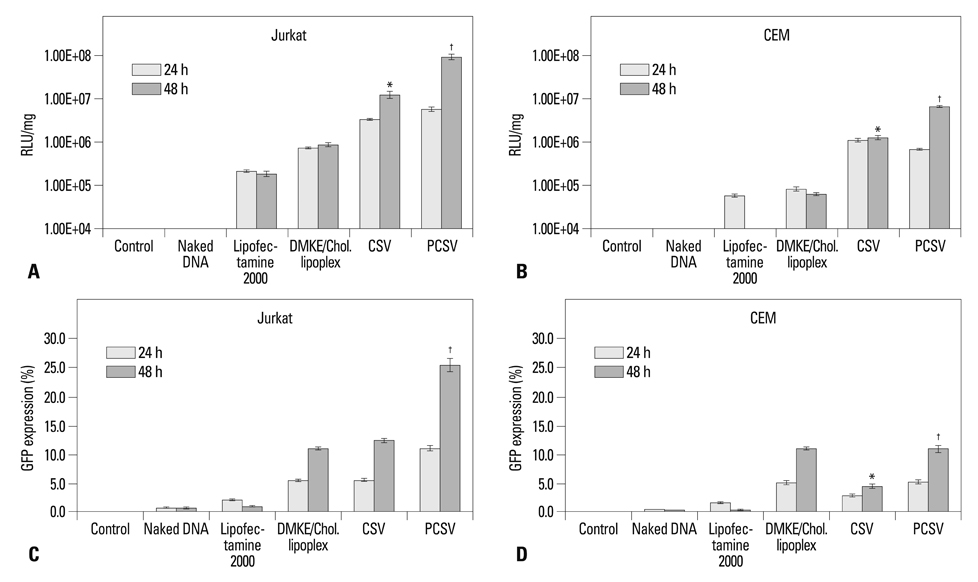
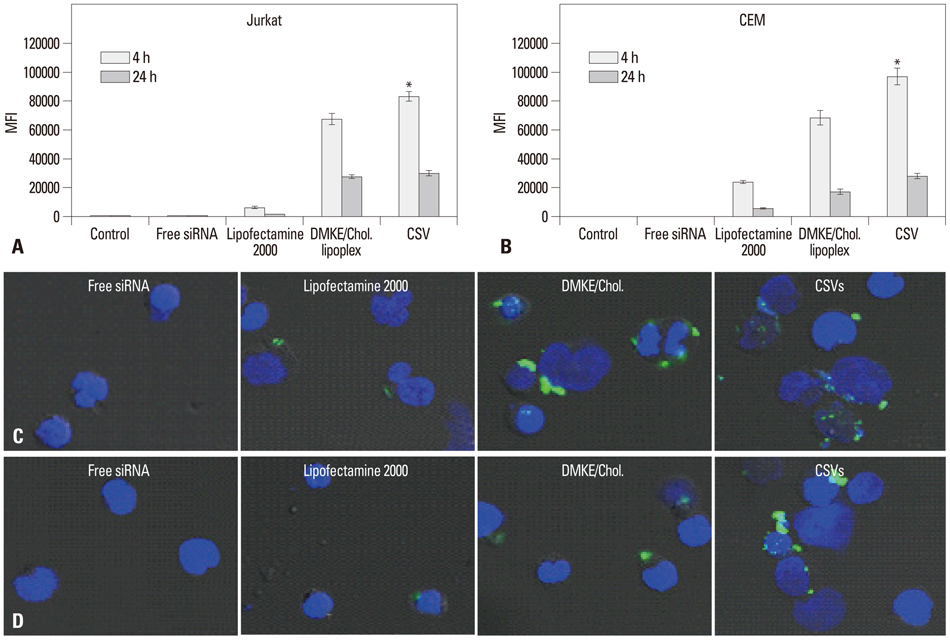
The optimized CSVs and PCSVs exhibited enhanced gene and siRNA delivery in the tested suspension cell lines (Jurkat cells and CEM cells), compared with conventional cationic liposomes. In the case of pDNA transfection, the CSVs and PCSVs show at least 10-fold and 100-fold higher transgene expression compared with DMKE lipoplexes (or lipofectamine 2000), respectively. The CSVs showed more effective siRNA delivery to the suspension cells than cationic liposomes, as assessed by confocal microscopy, FACS, and RT-PCR. The effective transfection by the CSVs and PCSVs is presumably due to fusogenic activity of F/HN proteins resulting in facilitated internalization of pDNA and siRNA.
CONCLUSION
This study suggests that Sendai F/HN viroplexes can be widely applicable for the transfection of pDNA and siRNA to suspension cell lines.
MeSH Terms
Figure
Reference
-
1. Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2009; 109:259–302.
Article2. Gao Y, Liu XL, Li XR. Research progress on siRNA delivery with nonviral carriers. Int J Nanomedicine. 2011; 6:1017–1025.
Article3. Larsen HØ, Roug AS, Nielsen K, Søndergaard CS, Hokland P. Nonviral transfection of leukemic primary cells and cells lines by siRNA-a direct comparison between Nucleofection and Accell delivery. Exp Hematol. 2011; 39:1081–1089.
Article4. Bagai S, Puri A, Blumenthal R, Sarkar DP. Hemagglutinin-neuraminidase enhances F protein-mediated membrane fusion of reconstituted Sendai virus envelopes with cells. J Virol. 1993; 67:3312–3318.
Article5. Kaneda Y. Update on non-viral delivery methods for cancer therapy: possibilities of a drug delivery system with anticancer activities beyond delivery as a new therapeutic tool. Expert Opin Drug Deliv. 2010; 7:1079–1093.
Article6. Khoshnejad M, Young PR, Toth I, Minchin RF. Modified influenza virosomes: recent advances and potential in gene delivery. Curr Med Chem. 2007; 14:3152–3156.
Article7. de Jonge J, Holtrop M, Wilschut J, Huckriede A. Reconstituted influenza virus envelopes as an efficient carrier system for cellular delivery of small-interfering RNAs. Gene Ther. 2006; 13:400–411.
Article8. Lund PE, Hunt RC, Gottesman MM, Kimchi-Sarfaty C. Pseudovirions as vehicles for the delivery of siRNA. Pharm Res. 2010; 27:400–420.
Article9. Tu Y, Kim JS. A fusogenic segment of glycoprotein H from herpes simplex virus enhances transfection efficiency of cationic liposomes. J Gene Med. 2008; 10:646–654.
Article10. Kim HS, Kim JS, Lee YK, Koo KH, Park YS. An efficient liposomal gene delivery vehicle using Sendai F/HN proteins and protamine. Cancer Gene Ther. 2008; 15:214–224.
Article11. Zhang Q, Li Y, Shi Y, Zhang Y. HVJ envelope vector, a versatile delivery system: its development, application, and perspectives. Biochem Biophys Res Commun. 2008; 373:345–349.
Article12. Sanderson CM, Avalos R, Kundu A, Nayak DP. Interaction of Sendai viral F, HN, and M proteins with host cytoskeletal and lipid components in Sendai virus-infected BHK cells. Virology. 1995; 209:701–707.
Article13. Kim HS, Moon J, Kim KS, Choi MM, Lee JE, Heo Y, et al. Gene-transferring efficiencies of novel diamino cationic lipids with varied hydrocarbon chains. Bioconjug Chem. 2004; 15:1095–1101.
Article14. Kim HS, Song IH, Kim JC, Kim EJ, Jang DO, Park YS. In vitro and in vivo gene-transferring characteristics of novel cationic lipids, DMKD (O,O'-dimyristyl-N-lysyl aspartate) and DMKE (O,O'-dimyristyl-N-lysyl glutamate). J Control Release. 2006; 115:234–241.
Article15. Altenburg JD, Harvey KA, McCray S, Xu Z, Siddiqui RA. A novel 2,6-diisopropylphenyl-docosahexaenoamide conjugate induces apoptosis in T cell acute lymphoblastic leukemia cell lines. Biochem Biophys Res Commun. 2011; 411:427–432.
Article16. Cron RQ, Schubert LA, Lewis DB, Hughes CC. Consistent transient transfection of DNA into non-transformed human and murine T-lymphocytes. J Immunol Methods. 1997; 205:145–150.
Article17. Hawley-Nelson P, Ciccarone V, Moore ML. Transfection of cultured eukaryotic cells using cationic lipid reagents. Curr Protoc Mol Biol. 2008; Chapter 9:Unit 9.4.18. Lee YK, Kim KS, Kim JS, Baek JE, Park SI, Jeong HY, et al. Leukemia-specific siRNA delivery by immunonanoplexes consisting of anti-JL1 minibody conjugated to oligo-9 Arg-peptides. Mol Cells. 2010; 29:457–462.
Article19. Fyrberg A, Lotfi K. Optimization and evaluation of electroporation delivery of siRNA in the human leukemic CEM cell line. Cytotechnology. 2010; 62:497–507.
Article20. Huckriede A, De Jonge J, Holtrop M, Wilschut J. Cellular delivery of siRNA mediated by fusion-active virosomes. J Liposome Res. 2007; 17:39–47.
Article21. Sorgi FL, Bhattacharya S, Huang L. Protamine sulfate enhances lipid-mediated gene transfer. Gene Ther. 1997; 4:961–968.
Article22. Hogrefe RI, Lebedev AV, Zon G, Pirollo KF, Rait A, Zhou Q, et al. Chemically modified short interfering hybrids (siHYBRIDS): nanoimmunoliposome delivery in vitro and in vivo for RNAi of HER-2. Nucleosides Nucleotides Nucleic Acids. 2006; 25:889–907.
Article23. Bramsen JB, Kjems J. Chemical modification of small interfering RNA. Methods Mol Biol. 2011; 721:77–103.
Article24. Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994; 63:345–382.
Article25. Schallon A, Synatschke CV, Jérôme V, Müller AH, Freitag R. Nanoparticulate nonviral agent for the effective delivery of pDNA and siRNA to differentiated cells and primary human T lymphocytes. Biomacromolecules. 2012; 13:3463–3474.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Efficient Gene Delivery into Human Mesenchymal Stem Cells Using Retroviral Vectors
- Generation of Leukemic Dendritic Cells from Patients with Acute Myelogenous Leukemia
- Efficiency of Chlamydia Pneumoniae Culture in the Upper Airway Epithelial Cell Lines: AMC-HN-4, AMC-HN-7, and AMC-HN-8
- Comparison of Various Transfection Methods in Human and Bovine Cultured Cells
- Cloning and Expression of Hemagglutinin-Neuraminidase Gene of a Thermostable Isolate of Newcastle Disease Virus by Baculovirus Recombinants