Korean J Ophthalmol.
2013 Dec;27(6):425-432. 10.3341/kjo.2013.27.6.425.
Anti-VEGF-refractory Exudative Age-related Macular Degeneration: Differential Response According to Features on Optical Coherence Tomography
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. sejoon1@hanmail.net
- KMID: 1792078
- DOI: http://doi.org/10.3341/kjo.2013.27.6.425
Abstract
- PURPOSE
To describe optical coherence tomography (OCT) characteristics of neovascular age-related macular degeneration (AMD) patients refractory to intravitreal anti-vascular endothelial growth factor (VEGF) injections (ranibizumab, bevacizumab) and their responses to alternative anti-VEGF agents or photodynamic therapy (PDT).
METHODS
A retrospective review of 267 neovascular AMD patients treated with intravitreal anti-VEGF injections.
RESULTS
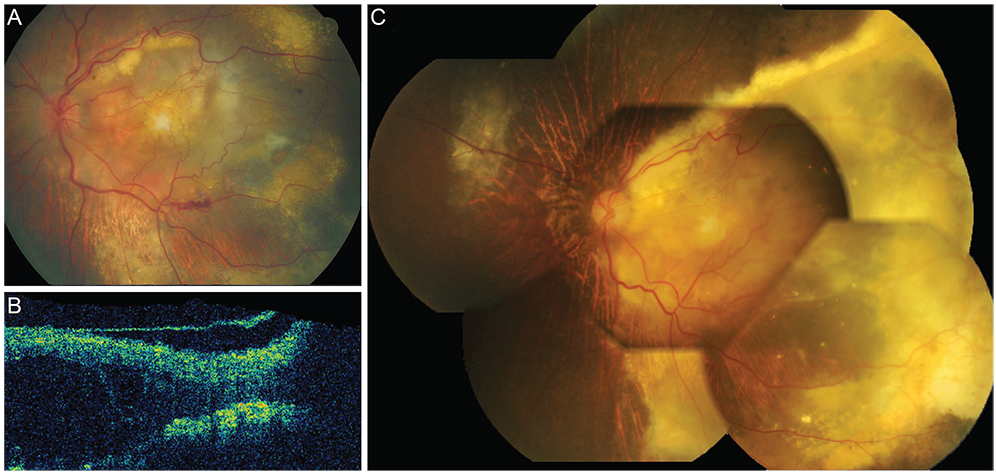
Twenty patients (7.5%) were refractory to anti-VEGF injections (stationary or increased retinal exudation despite three or more monthly injections). They were grouped into either the extensive intraretinal fluid group (IRF group, 9 patients) or the subretinal fluid only group (SRF group, 11 patients) according to OCT findings. In the IRF group, response rates to subsequent treatment were 0% (0 / 7) for bevacizumab, 50% (3 / 6) for ranibizumab and 50% (3 / 6) for PDT +/- anti-VEGF. Three out of four bevacizumab-refractory patients showed response to ranibizumab as a secondary treatment. In the SRF group, response rates were lower with 0% (0 / 7) for bevacizumab, 22.2% (2 / 9) for ranibizumab and 28.6% (2 / 7) for PDT +/- anti-VEGF. One out of four bevacizumab-refractory patients responded to ranibizumab. The visual outcome was worse in the IRF group (median 20 / 1,000) than in the SRF group (median 20 / 100).
CONCLUSIONS
In anti-VEGF-refractory neovascular AMD, patients with extensive IRF refractory to bevacizumab can be responsive to ranibizumab while patients with SRF may be refractory to both, suggesting a different pathophysiology and intraocular pharmacokinetics.
Keyword
MeSH Terms
-
Aged
Aged, 80 and over
Angiogenesis Inhibitors/administration & dosage
Antibodies, Monoclonal, Humanized/*administration & dosage
Female
Fluorescein Angiography
Follow-Up Studies
Fundus Oculi
Humans
Intravitreal Injections
Male
Middle Aged
Retrospective Studies
Tomography, Optical Coherence/*methods
Treatment Outcome
Vascular Endothelial Growth Factor A/*antagonists & inhibitors
Visual Acuity
Wet Macular Degeneration/*drug therapy/metabolism/pathology
Angiogenesis Inhibitors
Antibodies, Monoclonal, Humanized
Vascular Endothelial Growth Factor A
Figure
Reference
-
1. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–1431.2. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–1444.3. Abraham-Marin ML, Cortes-Luna CF, Alvarez-Rivera G, et al. Intravitreal bevacizumab therapy for neovascular age-related macular degeneration: a pilot study. Graefes Arch Clin Exp Ophthalmol. 2007; 245:651–655.4. Avery RL, Pieramici DJ, Rabena MD, et al. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006; 113:363–372.e5.5. Costa RA, Jorge R, Calucci D, et al. Intravitreal bevacizumab for choroidal neovascularization caused by AMD (IBeNA Study): results of a phase 1 dose-escalation study. Invest Ophthalmol Vis Sci. 2006; 47:4569–4578.6. Fong DS, Custis P, Howes J, Hsu JW. Intravitreal bevacizumab and ranibizumab for age-related macular degeneration a multicenter, retrospective study. Ophthalmology. 2010; 117:298–302.7. Rich RM, Rosenfeld PJ, Puliafito CA, et al. Short-term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina. 2006; 26:495–511.8. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007; 143:566–583.9. Brown DM, Regillo CD. Anti-VEGF agents in the treatment of neovascular age-related macular degeneration: applying clinical trial results to the treatment of everyday patients. Am J Ophthalmol. 2007; 144:627–637.10. Cho M, Barbazetto IA, Freund KB. Refractory neovascular age-related macular degeneration secondary to polypoidal choroidal vasculopathy. Am J Ophthalmol. 2009; 148:70–78.e1.11. Mojana F, Cheng L, Bartsch DU, et al. The role of abnormal vitreomacular adhesion in age-related macular degeneration: spectral optical coherence tomography and surgical results. Am J Ophthalmol. 2008; 146:218–227.12. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials--TAP report. Arch Ophthalmol. 1999; 117:1329–1345.13. Lux A, Llacer H, Heussen FM, Joussen AM. Non-responders to bevacizumab (Avastin) therapy of choroidal neovascular lesions. Br J Ophthalmol. 2007; 91:1318–1322.14. CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011; 364:1897–1908.15. Stangos AN, Gandhi JS, Nair-Sahni J, et al. Polypoidal choroidal vasculopathy masquerading as neovascular age-related macular degeneration refractory to ranibizumab. Am J Ophthalmol. 2010; 150:666–673.16. Gaudreault J, Fei D, Beyer JC, et al. Pharmacokinetics and retinal distribution of ranibizumab, a humanized antibody fragment directed against VEGF-A, following intravitreal administration in rabbits. Retina. 2007; 27:1260–1266.17. Kofoed-Enevoldsen A, Foyle WJ, Fernandez M, Yudkin JS. Evidence of impaired glomerular charge selectivity in nondiabetic subjects with microalbuminuria: relevance to cardiovascular disease. Arterioscler Thromb Vasc Biol. 1996; 16:450–454.18. Nishihara H. Studies on the ultrastructure of the inner limiting membrane of the retina: distribution of anionic sites in the inner limiting membrane of the retina. Nihon Ganka Gakkai Zasshi. 1991; 95:951–958.19. Bunt-Milam AH, Saari JC, Klock IB, Garwin GG. Zonulae adherentes pore size in the external limiting membrane of the rabbit retina. Invest Ophthalmol Vis Sci. 1985; 26:1377–1380.20. Kim H, Robinson SB, Csaky KG. FcRn receptor-mediated pharmacokinetics of therapeutic IgG in the eye. Mol Vis. 2009; 15:2803–2812.21. Heiduschka P, Fietz H, Hofmeister S, et al. Penetration of bevacizumab through the retina after intravitreal injection in the monkey. Invest Ophthalmol Vis Sci. 2007; 48:2814–2823.22. Shahar J, Avery RL, Heilweil G, et al. Electrophysiologic and retinal penetration studies following intravitreal injection of bevacizumab (Avastin). Retina. 2006; 26:262–269.23. Brasil OF, Smith SD, Galor A, et al. Predictive factors for short-term visual outcome after intravitreal triamcinolone acetonide injection for diabetic macular oedema: an optical coherence tomography study. Br J Ophthalmol. 2007; 91:761–765.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Optical Coherence Tomography Characteristics among Three Subtypes of Exudative Age-related Macular Degeneration
- Optical Coherence Tomography of Idiopathic Polypoidal Choroidal Vasculopathy
- High Dose Intravitreal Bevacizumab for Refractory Pigment Epithelial Detachment in Age-related Macular Degeneration
- Anatomical Characteristics of End-stage Exudative Age-related Macular Degeneration Refractory to Intravitreal Anti-vascular Endothelial Growth Factor Injection
- Longitudinal Changes in Retinal Nerve Fiber Layer Thickness after Intravitreal Anti-vascular Endothelial Growth Factor Therapy