J Bacteriol Virol.
2013 Mar;43(1):9-17. 10.4167/jbv.2013.43.1.9.
Cell Culture-based Influenza Vaccines as Alternatives to Egg-based Vaccines
- Affiliations
-
- 1Department of Microbiology, College of Medicine, Hallym University, Chuncheon, Gangwon-do, Korea. ms0392@hallym.ac.kr
- 2Center for Medical Science Research, College of Medicine, Hallym University, Chuncheon, Gangwon-do, Korea.
- KMID: 1782698
- DOI: http://doi.org/10.4167/jbv.2013.43.1.9
Abstract
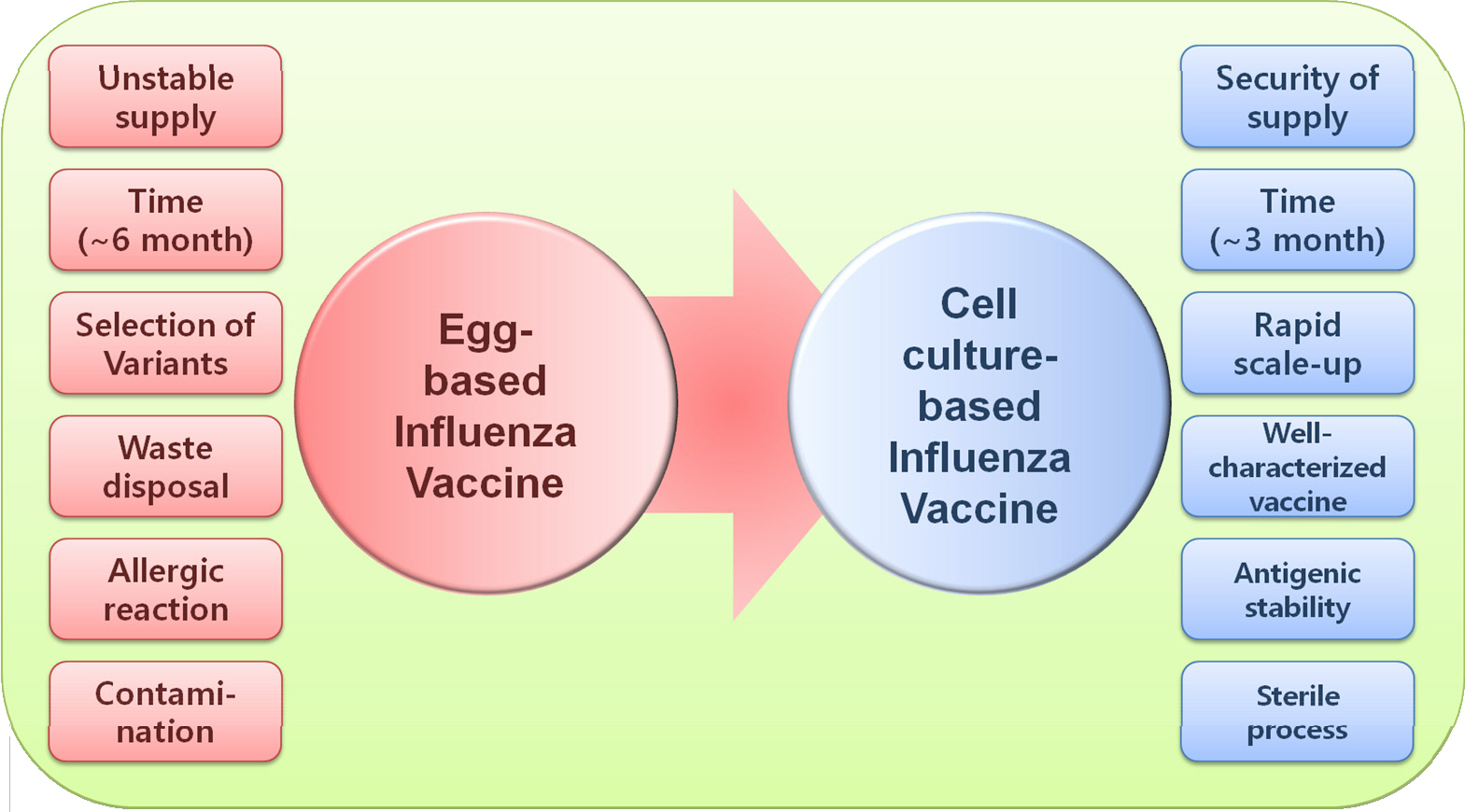
- Influenza viruses have raised public health concerns by seasonal epidemics and intermittent pandemics. Vaccination is considered as the most effective method for preventing influenza infection in humans. Current influenza vaccines are mostly produced in fertile chicken eggs. However, disadvantages of egg-based vaccines, such as egg dependency, labor-intensive manufacturing system, and huddle for large-scale output, allow us to make an alternative method. A cell-culture platform may be a fine alternative for the next generation vaccine technique. Compared with a classical egg-based method, cell-grown vaccines provide stable pipeline even in the pandemic situation with shorter lead-in times. In addition, cell-grown vaccines are flexible for altering production scales because stocked cell batches can be easily sub-cultured in large quantity without worrying avian diseases and a resultant decrease in egg production. By World Health Organization, MDCK, PER.C6, and Vero cells are only recommended for manufacturing influenza vaccines. In this review, we discuss the necessity, immunogenicity, and efficacy of cell-grown influenza vaccines compared with egg-based vaccines.
MeSH Terms
Figure
Cited by 2 articles
-
Strategy for Developing Medical Arsenals by Modulation of Membrane Fusion Activity of Influenza Virus Hemagglutinin
Sangmoo Lee, Jin Il Kim, Ilseob Lee, Man-Seong Park
J Bacteriol Virol. 2013;43(4):337-341. doi: 10.4167/jbv.2013.43.4.337.Is Obesity One of Physiological Factors which Exert Influenza Virus-induced Pathology and Vaccine Efficacy?
Whajung Cho, Jae-Hwan Nam
J Bacteriol Virol. 2014;44(3):226-235. doi: 10.4167/jbv.2014.44.3.226.
Reference
-
1). Lee I, Kim JI, Park MS. A Novel PA-X Protein Translated from Influenza A Virus Segment 3. J Bacteriol Virol. 2012; 42:368–71.
Article2). Tong S, Li Y, Rivailler P, Conrardy C, Castillo DA, Chen LM, et al. A distinct lineage of influenza A virus from bats. Proc Natl Acad Sci U S A. 2012; 109:4269–74.
Article3). WHO. Fact sheet on influenza. 2009.4). Klenk HD, Garten W, Matrosovich M. Molecular mechanisms of interspecies transmission and pathogenicity of influenza viruses: Lessons from the 2009 pandemic. Bioessays. 2011; 33:180–8.
Article5). WHO. Cumulative number of confirmed human cases of avian influenza A (H5N1) reported to WHO. 2012.6). Park S, Kim JI, Park MS. Antiviral Agents Against Influenza Viruses. J Bacteriol Virol. 2012; 42:284–93.
Article7). Cox NJ, Subbarao K. Influenza. Lancet. 1999; 354:1277–82.
Article8). Kim JI, Park MS. An Universal Approach to Getting Ahead for Influenza B Vaccines. J Bacteriol Virol. 2012; 42:363–7.
Article9). Traynor K. First quadrivalent flu vaccine approved. Am J Health Syst Pharm. 2012; 69:538.
Article10). WHO. A description of the process of seasonal and H5N1 influenza vaccine virus selection and development. 2007.11). Nicholson KG WR, Hay AJ. Textbook of Influenza. Oxford: Blackwell Science Ltd.;1998.12). WHO. Cell culture as a substrate for the production of influenza vaccines: memorandum from a WHO meeting. Bull World Health Organ. 1995; 73:431–5.13). Magrath DI. Safety of vaccines produced in continuous cell lines. Dev Biol Stand. 1991; 75:17–20.14). Merten OW, Hannoun C, Manuguerra JC, Ventre F, Petres S. Production of influenza virus in cell cultures for vaccine preparation. Adv Exp Med Biol. 1996; 397:141–51.
Article15). Tannock GA, Bryce DA, Paul JA. Evaluation of chicken kidney and chicken embryo kidney cultures for the large-scale growth of attenuated influenza virus master strain A/Ann/Arbor/6/60-ca. Vaccine. 1985; 3:333–9.
Article16). Kistner O, Barrett PN, Mundt W, Reiter M, Schober-Bendixen S, Dorner F. Development of a mammalian cell (Vero) derived candidate influenza virus vaccine. Vaccine. 1998; 16:960–8.
Article17). PATH. Influenza Vaccine Strategies for Broad Global Access. www.oliverwyman.com/media/VAC_infl_publ_rpt_10-07.pdf. 2007.18). Feng SZ, Jiao PR, Qi WB, Fan HY, Liao M. Development and strategies of cell-culture technology for influenza vaccine. Appl Microbiol Biotechnol. 2011; 89:893–902.
Article19). Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003; 21:1776–9.
Article20). WHO. World now at the start of 2009 influenza pandemic. 2009.21). Pandey A, Singh N, Sambhara S, Mittal SK. Egg-independent vaccine strategies for highly pathogenic H5N1 influenza viruses. Hum Vaccin. 2010; 6:178–88.
Article22). Robertson JS, Naeve CW, Webster RG, Bootman JS, Newman R, Schild GC. Alterations in the hemagglutinin associated with adaptation of influenza B virus to growth in eggs. Virology. 1985; 143:166–74.
Article23). Oxford JS, Schild GC, Corcoran T, Newman R, Major D, Robertson J, et al. A host-cell-selected variant of influenza B virus with a single nucleotide substitution in HA affecting a potential glycosylation site was attenuated in virulence for volunteers. Arch Virol. 1990; 110:37–46.24). Oxford JS, Corcoran T, Knott R, Bates J, Bartolomei O, Major D, et al. Serological studies with influenza A(H1N1) viruses cultivated in eggs or in a canine kidney cell line (MDCK). Bull World Health Organ. 1987; 65:181–7.25). Katz JM, Webster RG. Amino acid sequence identity between the HA1 of influenza A (H3N2) viruses grown in mammalian and primary chick kidney cells. J Gen Virol. 1992; 73:1159–65.
Article26). Katz JM, Wang M, Webster RG. Direct sequencing of the HA gene of influenza (H3N2) virus in original clinical samples reveals sequence identity with mammalian cell-grown virus. J Virol. 1990; 64:1808–11.
Article27). James JM, Zeiger RS, Lester MR, Fasano MB, Gern JE, Mansfield LE, et al. Safe administration of influenza vaccine to patients with egg allergy. J Pediatr. 1998; 133:624–8.
Article28). WHO. Use of Cell Lines for the Production of Influenza Virus Vaccines: An Appraisal of Technical, Manufacturing, and Regulatory Considerations. 2007.29). Barrett PN, Mundt W, Kistner O, Howard MK. Vero cell platform in vaccine production: moving towards cell culture-based viral vaccines. Expert Rev Vaccines. 2009; 8:607–18.
Article30). U.S. Health and Human Services. HHS Awards Contracts Totaling More Than $1 Billion To Develop Cell-Based Influenza Vaccine. 2006.31). U.S. Health and Human Services. HHS Awards $487 Million Contract to Build First U.S. Manufacturing Facility for Cell-Based Influenza Vaccine. 2009.32). Montagnon BJ, Fanget B, Nicolas AJ. The large-scale cultivation of VERO cells in micro-carrier culture for virus vaccine production. Preliminary results for killed poliovirus vaccine. Dev Biol Stand. 1981; 47:55–64.33). SanofiPasteur. Our vaccines: a history of innovation. www.sanofipasteur.com/index.jsp?codeRubrique=14&lang=EN.34). Montagnon BJ. Polio and rabies vaccines produced in continuous cell lines: a reality for Vero cell line. Dev Biol Stand. 1989; 70:27–47.35). Schuller E, Jilma B, Voicu V, Golor G, Kollaritsch H, Kaltenböck A, et al. Long-term immunogenicity of the new Vero cell-derived, inactivated Japanese encephalitis virus vaccine IC51 Six and 12 month results of a multicenter follow-up phase 3 study. Vaccine. 2008; 26:4382–6.36). Tauber E, Kollaritsch H, von Sonnenburg F, Lademann M, Jilma B, Firbas C, et al. Randomized, double-blind, placebo-controlled phase 3 trial of the safety and tolerability of IC51, an inactivated Japanese encephalitis vaccine. J Infect Dis. 2008; 198:493–9.
Article37). Tauber E, Kollaritsch H, Korinek M, Rendi-Wagner P, Jilma B, Firbas C, et al. Safety and immunogenicity of a Vero-cell-derived, inactivated Japanese encephalitis vaccine: a non-inferiority, phase III, randomised controlled trial. Lancet. 2007; 370:1847–53.
Article38). Lyons A, Kanesa-thasan N, Kuschner RA, Eckels KH, Putnak R, Sun W, et al. A Phase 2 study of a purified, inactivated virus vaccine to prevent Japanese encephalitis. Vaccine. 2007; 25:3445–53.
Article39). Dennehy PH. Rotavirus vaccines: an overview. Clin Microbiol Rev. 2008; 21:198–208.
Article40). Lau SC, Scholtissek C. Abortive infection of Vero cells by an influenza A virus (FPV). Virology. 1995; 212:225–31.
Article41). Nakamura K, Homma M. Protein synthesis in Vero cells abortively infected with influenza B virus. J Gen Virol. 1981; 56:199–202.
Article42). Govorkova EA, Kaverin NV, Gubareva LV, Meignier B, Webster RG. Replication of influenza A viruses in a green monkey kidney continuous cell line (Vero). J Infect Dis. 1995; 172:250–3.
Article43). Kaverin NV, Webster RG. Impairment of multicycle influenza virus growth in Vero (WHO) cells by loss of trypsin activity. J Virol. 1995; 69:2700–3.
Article44). Govorkova EA, Murti G, Meignier B, de Taisne C, Webster RG. African green monkey kidney (Vero) cells provide an alternative host cell system for influenza A and B viruses. J Virol. 1996; 70:5519–24.
Article45). Baxter. Baxter Receives European Commission Approval for CELVAPAN H1N1 Pandemic Influenza Vaccine. 2009.46). Baxter. Baxter Receives EMEA Positive Opinion for CELVAPAN, the First Cell Culture-based Pandemic Flu Vaccine. 2008.47). Minor PD, Engelhardt OG, Wood JM, Robertson JS, Blayer S, Colegate T, et al. Current challenges in implementing cell-derived influenza vaccines: implications for production and regulation, July 2007, NIBSC, Potters Bar, UK. Vaccine. 2009; 27:2907–13.
Article48). Tree JA, Richardson C, Fooks AR, Clegg JC, Looby D. Comparison of large-scale mammalian cell culture systems with egg culture for the production of influenza virus A vaccine strains. Vaccine. 2001; 19:3444–50.
Article49). Liu J, Shi X, Schwartz R, Kemble G. Use of MDCK cells for production of live attenuated influenza vaccine. Vaccine. 2009; 27:6460–3.
Article50). Genzel Y, Reichl U. Continuous cell lines as a production system for influenza vaccines. Expert Rev Vaccines. 2009; 8:1681–92.
Article51). Liu J, Mani S, Schwartz R, Richman L, Tabor DE. Cloning and assessment of tumorigenicity and oncogenicity of a Madin-Darby canine kidney (MDCK) cell line for influenza vaccine production. Vaccine. 2010; 28:1285–93.
Article52). Novartis. Novartis receives FDA approval for Flucelvax®, the first cell-culture vaccine in US to help protect against seasonal influenza. 2012.53). Pau MG, Ophorst C, Koldijk MH, Schouten G, Mehtali M, Uytdehaag F. The human cell line PER.C6 provides a new manufacturing system for the production of influenza vaccines. Vaccine. 2001; 19:2716–21.
Article54). Cox RJ, Madhun AS, Hauge S, Sjursen H, Major D, Kuhne M, et al. A phase I clinical trial of a PER.C6 cell grown influenza H7 virus vaccine. Vaccine. 2009; 27:1889–97.
Article55). Ledwith BJ, Lanning CL, Gumprecht LA, Anderson CA, Coleman JB, Gatto NT, et al. Tumorigenicity assessments of Per.C6 cells and of an Ad5-vectored HIV-1 vaccine produced on this continuous cell line. Dev Biol (Basel). 2006; 123:251–63.56). Zhang W, Xue T, Wu X, Zhang P, Zhao G, Peng D, et al. Increase in viral yield in eggs and MDCK cells of reassortant H5N1 vaccine candidate viruses caused by insertion of 38 amino acids into the NA stalk. Vaccine. 2011; 29:8032–41.
Article57). Tseng YF, Hu AY, Huang ML, Yeh WZ, Weng TC, Chen YS, et al. Adaptation of high-growth influenza H5N1 vaccine virus in Vero cells: implications for pandemic preparedness. PLoS One. 2011; 6:e24057.
Article58). Murakami S, Horimoto T, Ito M, Takano R, Katsura H, Shimojima M, et al. Enhanced growth of influenza vaccine seed viruses in vero cells mediated by broadening the optimal pH range for virus membrane fusion. J Virol. 2012; 86:1405–10.
Article59). Szymczakiewicz-Multanowska A, Groth N, Bugarini R, Lattanzi M, Casula D, Hilbert A, et al. Safety and immunogenicity of a novel influenza subunit vaccine produced in mammalian cell culture. J Infect Dis. 2009; 200:841–8.
Article60). Reisinger KS, Block SL, Izu A, Groth N, Holmes SJ. Subunit influenza vaccines produced from cell culture or in embryonated chicken eggs: comparison of safety, reactogenicity, and immunogenicity. J Infect Dis. 2009; 200:849–57.
Article61). Groth N, Montomoli E, Gentile C, Manini I, Bugarini R, Podda A. Safety, tolerability and immunogenicity of a mammalian cell-culture-derived influenza vaccine: a sequential Phase I and Phase II clinical trial. Vaccine. 2009; 27:786–91.
Article62). Hatz C, Cramer JP, Vertruyen A, Schwarz TF, von Sonnenburg F, Borkowski A, et al. A randomised, single-blind, dose-range study to assess the immunogenicity and safety of a cell-culture-derived A/H1N1 influenza vaccine in adult and elderly populations. Vaccine. 2012; 30:4820–7.
Article63). Ehrlich HJ, Müller M, Oh HM, Tambyah PA, Joukhadar C, Montomoli E, et al. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med. 2008; 358:2573–84.
Article