Infect Chemother.
2013 Dec;45(4):375-386. 10.3947/ic.2013.45.4.375.
Influenza Vaccines: Unmet Needs and Recent Developments
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. wjkim@korea.ac.kr
- 2Asian Pacific Influenza Institute, Korea University College of Medicine, Seoul, Korea.
- 3Transgovernmental Enterprise for Pandemic Influenza in Korea, Seoul, Korea.
- KMID: 2170441
- DOI: http://doi.org/10.3947/ic.2013.45.4.375
Abstract
- Influenza is a worldwide public health concern. Since the introduction of trivalent influenza vaccine in 1978, vaccination has been the primary means of prevention and control of influenza. Current influenza vaccines have moderate efficacy, good safety, and acceptable tolerability; however, they have unsatisfactory efficacy in older adults, are dependent on egg supply for production, and are time-consuming to manufacture. This review outlines the unmet medical needs of current influenza vaccines. Recent developments in influenza vaccines are also described.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Revised Adult Immunization Guideline Recommended by the Korean Society of Infectious Diseases, 2014
Won Suk Choi, Jung-Hyun Choi, Ki Tae Kwon, Kyung Seo, Min A Kim, Sang-Oh Lee, Young Jin Hong, Jin-Soo Lee, Joon Young Song, Ji Hwan Bang, Hee-Jung Choi, Young-Hwa Choi, Dong Gun Lee, Hee Jin Cheong, ,
Infect Chemother. 2015;47(1):68-79. doi: 10.3947/ic.2015.47.1.68.Vaccination for Diabetic Patients: An Update
Joon-Sup Yeom
J Korean Diabetes. 2015;16(4):236-241. doi: 10.4093/jkd.2015.16.4.236.
Reference
-
1. Hilleman MR. Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control. Vaccine. 2002; 20:3068–3087.
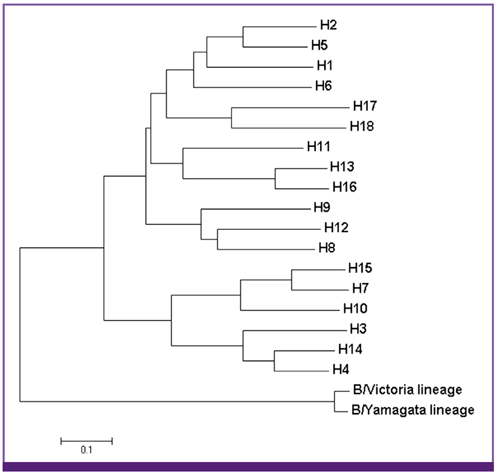
Article2. McCullers JA, Saito T, Iverson AR. Multiple genotypes of influenza B virus circulated between 1979 and 2003. J Virol. 2004; 78:12817–12828.
Article3. Smith W, Andrewes CH, Laidlaw PP. A virus obtained from influenza patients. Lancet. 1933; 2:66–68.
Article4. Francis T Jr. A new type of virus from epidemic influenza. Science. 1940; 92:405–408.
Article5. Cox NJ, Subbarao K. Influenza. Lancet. 1999; 354:1277–1282.
Article6. Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, Cheng PY, Bandaranayake D, Breiman RF, Brooks WA, Buchy P, Feikin DR, Fowler KB, Gordon A, Hien NT, Horby P, Huang QS, Katz MA, Krishnan A, Lal R, Montgomery JM, Mølbak K, Pebody R, Presanis AM, Razuri H, Steens A, Tinoco YO, Wallinga J, Yu H, Vong S, Bresee J, Widdowson MA. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis. 2012; 12:687–695.
Article7. Programme WHOGI. Pandemic influenza preparedness and response: a WHO guidance document: World Health Organization. 2009.8. Team DPIP. UK Influenza pandemic preparedness strategy. 2011.9. Kageyama S. Pandemic influenza: a never-ending story. Yonago Acta Med. 2011; 54:41–48.10. Simonsen L, Spreeuwenberg P, Lustig R, Taylor RJ, Fleming DM, Kroneman M, Van Kerkhove MD, Mounts AW, Paget WJ. GLaMOR Collaborating Teams. Global Mortality Estimates for the 2009 Influenza Pandemic from the GLaMOR Project : A Model ing Study. PLoS Med. 2013; 10:e1001558.11. WHO. Vaccines: Influenza. Accessed 19 November 2013. Available at: http://www.who.int/biologicals/vaccines/influenza/en/.12. Terebuh P, Uyeki T, Fukuda K. Impact of influenza on young children and the shaping of United States influenza vaccine policy. Pediatr Infect Dis J. 2003; 22:10 Suppl. S231–S235.
Article13. Centers for Disease Control and Prevention (CDC). Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices--United States, 2013-2014. MMWR Recomm Rep. 2013; 62:1–43.14. Hannoun C. The evolving history of influenza viruses and influenza vaccines. Expert Rev Vaccines. 2013; 12:1085–1094.
Article15. Partridge J, Kieny MP. Global production capacity of seasonal influenza vaccine in 2011. Vaccine. 2013; 31:728–731.
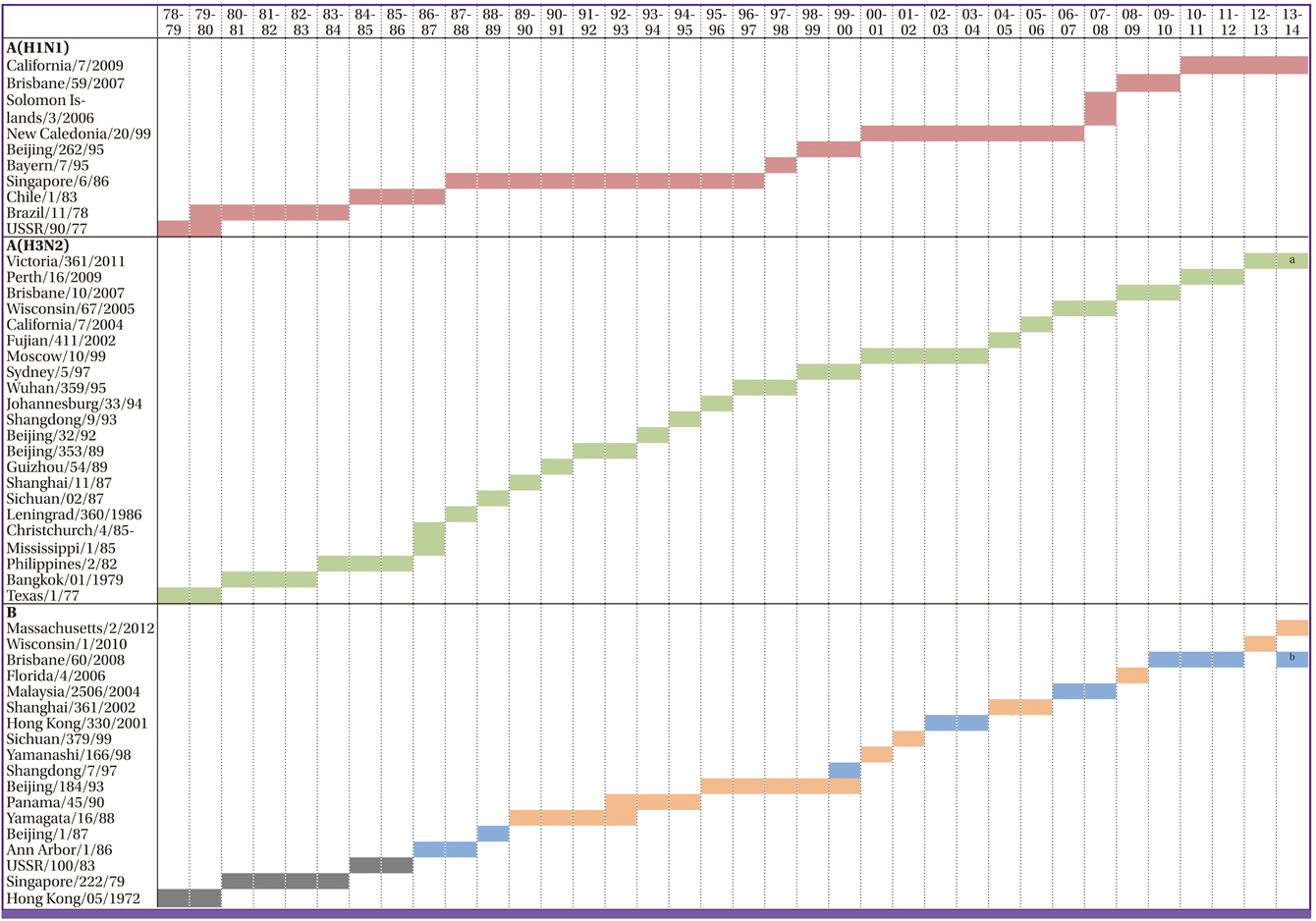
Article16. WHO. WHO recommendations on the composition of influenza virus vaccines. Accessed 19 November 2013. Available at: http://www.who.int/influenza/vaccines/virus/recommendations/en/.17. Richard SA, Viboud C, Miller MA. Evaluation of Southern Hemisphere influenza vaccine recommendations. Vaccine. 2010; 28:2693–2699.
Article18. Influenza research database. World health organization recommendations for composition of influenza vaccines. Accessed 16 December 2013. Available at: http://www.fludb.org/brc/vaccineRecommend.do?decorator=influenza.19. Nichol KL. Efficacy and effectiveness of influenza vaccination. Vaccine. 2008; 26:Suppl 4. D17–D22.
Article20. DiazGranados CA, Denis M, Plotkin S. Seasonal influenza vaccine efficacy and its determinants in children and non-elderly adults: a systematic review with meta-analyses of controlled trials. Vaccine. 2012; 31:49–57.
Article21. Jefferson TO, Rivetti D, Di Pietrantonj C, Rivetti A, Demicheli V. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2007; CD001269.22. Edwards KM, Dupont WD, Westrich MK, Plummer WD Jr, Palmer PS, Wright PF. A randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J Infect Dis. 1994; 169:68–76.
Article23. Choi WS, Noh JY, Seo YB, Baek JH, Lee J, Song JY, Park DW, Lee JS, Cheong HJ, Kim WJ. Case-control study of the effectiveness of the 2010-2011 seasonal influenza vaccine for prevention of laboratory-confirmed influenza virus infection in the Korean adult population. Clin Vaccine Immunol. 2013; 20:877–881.
Article24. Beyer WE, McElhaney J, Smith DJ, Monto AS, Nguyen-Van-Tam JS, Osterhaus AD. Cochrane re-arranged: Support for policies to vaccinate elderly people against influenza. Vaccine. 2013; 31:6030–6033.
Article25. Vu T, Farish S, Jenkins M, Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002; 20:1831–1836.
Article26. Nichol KL, Margolis KL, Wuorenma J, Von Sternberg T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N Engl J Med. 1994; 331:778–784.
Article27. Carrat F, Flahault A. Influenza vaccine: the challenge of antigenic drift. Vaccine. 2007; 25:6852–6862.
Article28. Nordin J, Mullooly J, Poblete S, Strikas R, Petrucci R, Wei F, Rush B, Safirstein B, Wheeler D, Nichol KL. Influenza vaccine effectiveness in preventing hospitalizations and deaths in persons 65 years or older in Minnesota, New York, and Oregon: data from 3 health plans. J Infect Dis. 2001; 184:665–670.
Article29. Ambrose CS, Wu X, Jones T, Mallory RM. The role of nasal IgA in children vaccinated with live attenuated influenza vaccine. Vaccine. 2012; 30:6794–6801.
Article30. Meadows M. Nasal flu vaccine approved. FDA Consum. 2003; 37:27.
Article31. Ambrose CS, Levin MJ, Belshe RB. The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults. Influenza Other Respir Viruses. 2011; 5:67–75.
Article32. Longini IM, Halloran ME, Nizam A, Wolff M, Mendelman PM, Fast PE, Belshe RB. Estimation of the efficacy of live, attenuated influenza vaccine from a two-year, multi-center vaccine trial: implications for influenza epidemic control. Vaccine. 2000; 18:1902–1909.
Article33. Belshe RB, Mendelman PM, Treanor J, King J, Gruber WC, Piedra P, Bernstein DI, Hayden FG, Kotloff K, Zangwill K, Iacuzio D, Wolff M. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenzavirus vaccine in children. N Engl J Med. 1998; 338:1405–1412.
Article34. Belshe RB, Gruber WC. Prevention of otitis media in children with live attenuated influenza vaccine given intranasally. Pediatr Infect Dis J. 2000; 19:5 Suppl. S66–S71.
Article35. Monto AS, Ohmit SE, Petrie JG, Johnson E, Truscon R, Teich E, Rotthoff J, Boulton M, Victor JC. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med. 2009; 361:1260–1267.
Article36. Prosser LA, Meltzer MI, Fiore A, Epperson S, Bridges CB, Hinrichsen V, Lieu TA. Effects of adverse events on the projected population benefits and cost-effectiveness of using live attenuated influenza vaccine in children aged 6 months to 4 years. Arch Pediatr Adolesc Med. 2011; 165:112–118.
Article37. Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013; 19:1597–1608.
Article38. Salk JE, Laurent AM, Bailey ML. Direction of research on vaccination against influenza; new studies with immunologic adjuvants. Am J Public Health Nations Health. 1951; 41:669–677.
Article39. Salk JE, Laurent AM. The use of adjuvants in studies on influenza immunization. I. Measurements in monkeys of the dimensions of antigenicity of virus-mineral oil emulsions. J Exp Med. 1952; 95:429–447.40. Herbert WJ, Selwyn S, Philp JR. Field trials of adjuvant and saline influenza vaccines. Br J Prev Soc Med. 1965; 19:97–100.
Article41. . Antibody responses and clinical reactions with saline and oil adjuvant influenza virus vaccines. Br Med J. 1955; 2:1229–1232.42. Stuart-Harris CH. Adjuvant influenza vaccines. Bull World Health Organ. 1969; 41:617–621.43. Davis DJ, Philip RN, Bell JA, Vogel JE, Jensen DV. Epidemiologic studies on influenza in familial and general population observations. Am J Hyg. 1961; 73:138–147.
Article44. Tetsutani K, Ishii KJ. Adjuvants in influenza vaccines. Vaccine. 2012; 30:7658–7661.
Article45. Principi N, Esposito S. Adjuvanted influenza vaccines. Hum Vaccin Immunother. 2012; 8:59–66.
Article46. Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004; 82:497–505.
Article47. Martin JT. Development of an adjuvant to enhance the immune response to influenza vaccine in the elderly. Biologicals. 1997; 25:209–213.
Article48. Tsai TF. Fluad®-MF59®-adjuvanted influenza vaccine in older adults. Infect Chemother. 2013; 45:159–174.
Article49. Banzhoff A, Nacci P, Podda A. A new MF59-adjuvanted influenza vaccine enhances the immune response in the elderly with chronic diseases: results from an immunogenicity meta-analysis. Gerontology. 2003; 49:177–184.
Article50. Squarcione S, Sgricia S, Biasio LR, Perinetti E. Comparison of the reactogenicity and immunogenicity of a split and a subunit-adjuvanted influenza vaccine in elderly subjects. Vaccine. 2003; 21:1268–1274.
Article51. Podda A. The adjuvanted influenza vaccines with novel adjuvants: experience with the MF59-adjuvanted vaccine. Vaccine. 2001; 19:2673–2680.
Article52. Ansaldi F, Canepa P, Parodi V, Bacilieri S, Orsi A, Compagnino F, Icardi G, Durando P. Adjuvanted seasonal influenza vaccines and perpetual viral metamorphosis: the importance of cross-protection. Vaccine. 2009; 27:3345–3348.
Article53. Del Giudice G, Hilbert AK, Bugarini R, Minutello A, Popova O, Toneatto D, Schoendorf I, Borkowski A, Rappuoli R, Podda A. An MF59-adjuvanted inactivated influenza vaccine containing A/Panama/1999 (H3N2) induced broader serological protection against heterovariant influenza virus strain A/Fujian/2002 than a subunit and a split influenza vaccine. Vaccine. 2006; 24:3063–3065.
Article54. Ansaldi F, Bacilieri S, Durando P, Sticchi L, Valle L, Montomoli E, Icardi G, Gasparini R, Crovari P. Cross-protection by MF59-adjuvanted influenza vaccine: neutralizing and haemagglutination-inhibiting antibody activity against A(H3N2) drifted influenza viruses. Vaccine. 2008; 26:1525–1529.
Article55. Van Buynder PG, Konrad S, Van Buynder JL, Brodkin E, Krajden M, Ramler G, Bigham M. The comparative effectiveness of adjuvanted and unadjuvanted trivalent inactivated influenza vaccine (TIV) in the elderly. Vaccine. 2013; 31:6122–6128.
Article56. Mannino S, Villa M, Apolone G, Weiss NS, Groth N, Aquino I, Boldori L, Caramaschi F, Gattinoni A, Malchiodi G, Rothman KJ. Effectiveness of adjuvanted influenza vaccination in elderly subjects in northern Italy. Am J Epidemiol. 2012; 176:527–533.
Article57. Song JY, Cheong HJ, Noh JY, Seo YB, Choi WS, Cho GJ, Hwang TG, Kim WJ. Long-term and cross-reactive immunogenicity of inactivated trivalent influenza vaccine in the elderly: MF59-adjuvanted vaccine versus unadjuvanted vaccine. J Med Virol. 2013; 85:1591–1597.
Article58. McElhaney JE, Beran J, Devaster JM, Esen M, Launay O, Leroux-Roels G, Ruiz-Palacios GM, van Essen GA, Caplanusi A, Claeys C, Durand C, Duval X, El Idrissi M, Falsey AR, Feldman G, Frey SE, Galtier F, Hwang SJ, Innis BL, Kovac M, Kremsner P, McNeil S, Nowakowski A, Richardus JH, Trofa A, Oostvogels L. Influence65 study group. AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: a phase 3 randomised trial. Lancet Infect Dis. 2013; 13:485–496.
Article59. Koutsonanos DG, Compans RW, Skountzou I. Targeting the skin for microneedle delivery of influenza vaccine. Adv Exp Med Biol. 2013; 785:121–132.
Article60. Nicolas JF, Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines. 2008; 7:1201–1214.
Article61. Durando P, Iudici R, Alicino C, Alberti M, de Florentis D, Ansaldi F, Icardi G. Adjuvants and alternative routes of administration towards the development of the ideal influenza vaccine. Hum Vaccin. 2011; 7:Suppl. 29–40.
Article62. Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, Howe BJ, Dubin G. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004; 351:2286–2294.
Article63. Jo YM, Song JY, Hwang IS, Lee J, Oh SC, Kim JS, Kim SR, Kim WJ, Cheong HJ. Dose sparing strategy with intradermal influenza vaccination in patients with solid cancer. J Med Virol. 2009; 81:722–727.
Article64. Beran J, Ambrozaitis A, Laiskonis A, Mickuviene N, Bacart P, Calozet Y, Demanet E, Heijmans S, Van Belle P, Weber F, Salamand C. Intradermal influenza vaccination of healthy adults using a new microinjection system: a 3-year randomised controlled safety and immunogenicity trial. BMC Med. 2009; 7:13.
Article65. Arnou R, Icardi G, De Decker M, Ambrozaitis A, Kazek MP, Weber F, Van Damme P. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine. 2009; 27:7304–7312.
Article66. Durando P, Alicino C, Alberti M, Sticchi L, Turello V, Marensi L, Caiazzo AL, Panico MG, Giugliano F, Parlato A, Peluso F, Sgricia S, Icardi G. Italian Intradermal Influenza Vaccine Working Group. Acceptance and safety of the intradermal influenza vaccine among the elderly in Italy: an on-field national study. Adv Ther. 2012; 29:312–326.
Article67. Marra F, Young F, Richardson K, Marra CA. A meta-analysis of intradermal versus intramuscular influenza vaccines: immunogenicity and adverse events. Influenza Other Respir Viruses. 2013; 7:584–603.
Article68. Kis EE, Winter G, Myschik J. Devices for intradermal vaccination. Vaccine. 2012; 30:523–538.
Article69. Duggan ST, Plosker GL. Intanza 15 mug intradermal seasonal influenza vaccine in older adults (aged ≥60 years): profile report. BioDrugs. 2010; 24:407–409.
Article70. Moro PL, Harrington T, Shimabukuro T, Cano M, Museru OI, Menschik D, Broder K. Adverse events after Fluzone® Intradermal vaccine reported to the Vaccine Adverse Event Reporting System (VAERS), 2011-2013. Vaccine. 2013; 31:4984–4987.
Article71. Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis. 2009; 200:172–180.
Article72. Tsang P, Gorse GJ, Strout CB, Sperling M, Greenberg DP, Ozol-Godfrey A, Diazgranados C, Landolfi V. Immunogenicity and safety of Fluzone® intradermal and high-dose influenza vaccines in older adults ≥65 years of age: A randomized, controlled, phase II trial. Vaccine. 2013; pii S0264-410X.
Article73. McKittrick N, Frank I, Jacobson JM, White CJ, Kim D, Kappes R, DiGiorgio C, Kenney T, Boyer J, Tebas P. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Ann Intern Med. 2013; 158:19–26.
Article74. Sullivan SJ, Jacobson R, Poland GA. Advances in the vaccination of the elderly against influenza: role of a high-dose vaccine. Expert Rev Vaccines. 2010; 9:1127–1133.
Article75. DiazGranados CA, Dunning AJ, Jordanov E, Landolfi V, Denis M, Talbot HK. High-dose trivalent influenza vaccine compared to standard dose vaccine in elderly adults: safety, immunogenicity and relative efficacy during the 2009-2010 season. Vaccine. 2013; 31:861–866.
Article76. Hess RD, Weber F, Watson K, Schmitt S. Regulatory, biosafety and safety challenges for novel cells as substrates for human vaccines. Vaccine. 2012; 30:2715–2727.
Article77. Doroshenko A, Halperin SA. Trivalent MDCK cell culture-derived influenza vaccine Optaflu® (Novartis Vaccines). Expert Rev Vaccines. 2009; 8:679–688.
Article78. Reynales H, Astudillo P, de Vallière S, Hatz C, Schlagenhauf P, Rath B, Velentgas P, Fariña A, Sales-Carmona V, Groth N. A prospective observational safety study on MF59® adjuvanted cell culture-derived vaccine, Celtura® during the A/H1N1 (2009) influenza pandemic. Vaccine. 2012; 30:6436–6443.
Article79. Barrett PN, Berezuk G, Fritsch S, Aichinger G, Hart MK, El-Amin W, Kistner O, Ehrlich HJ. Efficacy, safety, and immunogenicity of a Vero-cell-culture-derived trivalent influenza vaccine: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2011; 377:751–759.
Article80. Chan CY, Tambyah PA. Preflucel®: a Vero-cell culture-derived trivalent influenza vaccine. Expert Rev Vaccines. 2012; 11:759–773.
Article81. Vinnemeier CD, Fischer-Herr J, Meyer S, Liebig K, Theeß W, Burchard GD, Cramer JP. Immunogenicity and safety of an inactivated 2012/2013 trivalent influenza vaccine produced in mammalian cell culture (Optaflu®): An open label, uncontrolled study. Hum Vaccin Immunother. 2013; [In press].82. Frey S, Vesikari T, Szymczakiewicz-Multanowska A, Lattanzi M, Izu A, Groth N, Holmes S. Clinical efficacy of cell culture-derived and egg-derived inactivated subunit influenza vaccines in healthy adults. Clin Infect Dis. 2010; 51:997–1004.
Article83. Belshe RB. The need for quadrivalent vaccine against seasonal influenza. Vaccine. 2010; 28:Suppl 4. D45–D53.
Article84. Ambrose CS, Levin MJ. The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother. 2012; 8:81–88.
Article85. Kieninger D, Sheldon E, Lin WY, Yu CJ, Bayas JM, Gabor JJ, Esen M, Fernandez Roure JL, Narejos Perez S, Alvarez Sanchez C, Feng Y, Claeys C, Peeters M, Innis BL, Jain V. Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: a phase III, randomized trial in adults aged ≥18 years. BMC Infect Dis. 2013; 13:343.86. McKeage K. Inactivated quadrivalent split-virus seasonal influenza vaccine (Fluarix® quadrivalent): a review of its use in the prevention of disease caused by influenza A and B. Drugs. 2013; 73:1587–1594.
Article87. Pépin S, Donazzolo Y, Jambrecina A, Salamand C, Saville M. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine in adults. Vaccine. 2013; 31:5572–5578.
Article88. Goldenberg MM. Pharmaceutical approval update. P T. 2013; 38:150–152.89. Lambert LC, Fauci AS. Influenza vaccines for the future. N Engl J Med. 2010; 363:2036–2044.
Article90. Yang LP. Recombinant trivalent influenza vaccine (Flublok®): a review of its use in the prevention of seasonal influenza in adults. Drugs. 2013; 73:1357–1366.
Article91. Treanor JJ, El Sahly H, King J, Graham I, Izikson R, Kohberger R, Patriarca P, Cox M. Protective efficacy of a trivalent recombinant hemagglutinin protein vaccine (Flu-Blok®) against influenza in healthy adults: a randomized, placebo-controlled trial. Vaccine. 2011; 29:7733–7739.
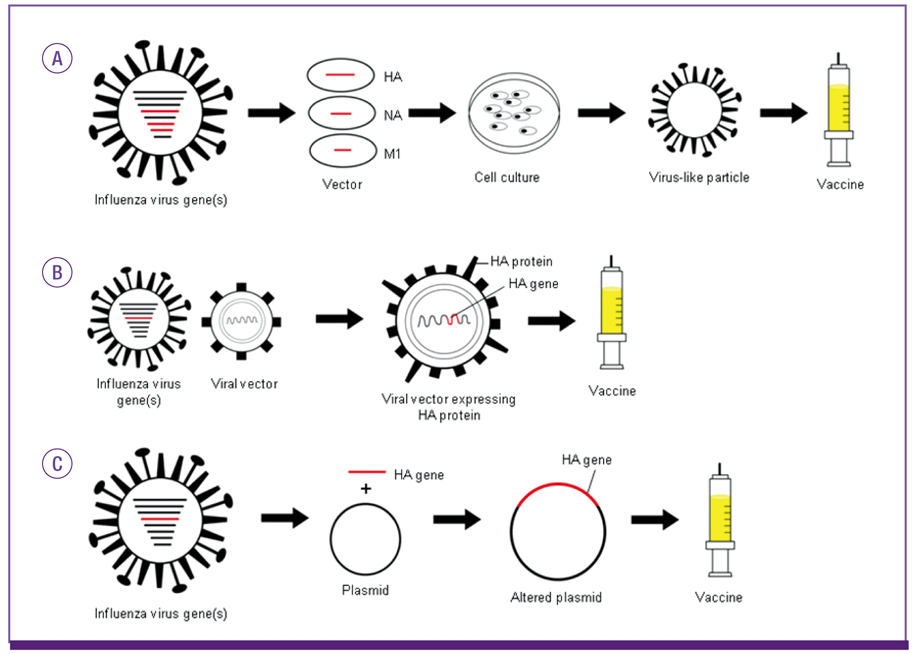
Article92. Dormitzer PR, Tsai TF, Del Giudice G. New technologies for influenza vaccines. Hum Vaccin Immunother. 2012; 8:45–58.
Article93. Ogunremi O, Pasick J, Kobinger GP, Hannaman D, Berhane Y, Clavijo A, van Drunen Littel-van den Hurk S. A single electroporation delivery of a DNA vaccine containing the hemagglutinin gene of Asian H5N1 avian influenza virus generated a protective antibody response in chickens against a North American virus strain. Clin Vaccine Immunol. 2013; 20:491–500.
Article94. Li J, Jiang Y, Zhao S, Chang X, Liu J, Zeng X, Li Y, Chen H. Protective efficacy of an H5N1 DNA vaccine against challenge with a lethal H5N1 virus in quail. Avian Dis. 2012; 56:4 Suppl. 937–939.
Article95. Ellebedy AH, Webby RJ. Influenza vaccines. Vaccine. 2009; 27:Suppl 4. D65–D68.
Article96. Shaw A. New technologies for new influenza vaccines. Vaccine. 2012; 30:4927–4933.
Article97. Pica N, Palese P. Toward a universal influenza virus vaccine: prospects and challenges. Annu Rev Med. 2013; 64:189–202.
Article98. Goodman AG, Heinen PP, Guerra S, Vijayan A, Sorzano CO, Gomez CE, Esteban M. A human multi-epitope recombinant vaccinia virus as a universal T cell vaccine candidate against influenza virus. PLoS One. 2011; 6:e25938.
Article