J Korean Med Sci.
2005 Feb;20(1):56-60. 10.3346/jkms.2005.20.1.56.
Expression of Inducible Nitric Oxide Synthase Is Increased in Rat Barrett's Esophagus Induced by Duodenal Contents Reflux
- Affiliations
-
- 1Department of Surgery, Dongguk University College of Medicine, Kyoungju, Korea. jong-dals@hanmail.net
- 2Department of Pathology, Dongguk University College of Medicine, Kyoungju, Korea.
- KMID: 1781725
- DOI: http://doi.org/10.3346/jkms.2005.20.1.56
Abstract
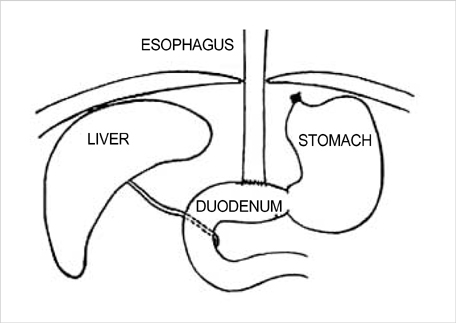
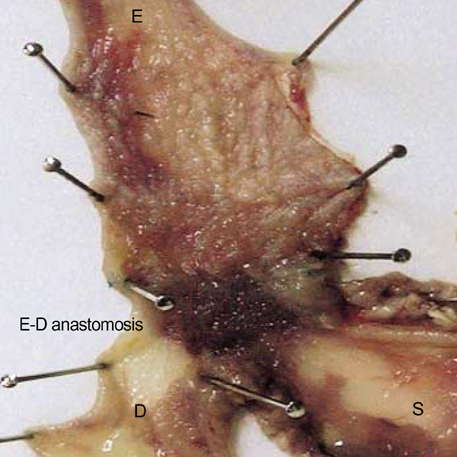
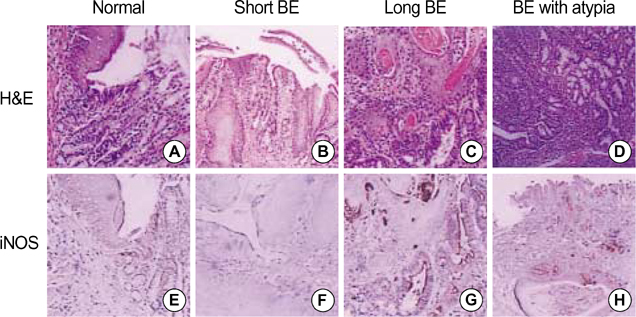
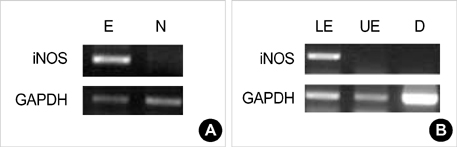
- Barrett's esophagus is a premalignant condition of esophageal adenocarcinoma. Inducible nitric oxide synthase (iNOS) is induced by cytokines and can generate locally high concentrations of nitric oxide (NO), whose metabolites can mediate genotoxicity and influence multistage carcinogenesis by causing DNA damage. Therefore, we evaluated the immunolocalization and expression of iNOS in surgically induced rat Barrett's esophagus. Esophagoduodenal anastomosis was performed in rats for inducing reflux of duodenal contents. Rats were killed at postoperative 10, 20, 30 and 40 weeks. We examined histologic changes and iNOS expression in esophagus by immunohistochemistry and reverse transcription-poly-merase chain reaction. Eighty six percent of experimental rats showed Barrett's esophagus above esophagoduodenal junction. iNOS immunoreactivity was clearly observed in the epithelial cells of Barrett's esophagus, predominantly at the apical surface of epithelial cells. Cytoplasmic staining was also seen only in atypical Barrett's esophagus. iNOS mRNA was detected only in the lower esophagus of experimental group. In conclusion, this study suggests that iNOS has some roles on Barrett's esophagus formation.
MeSH Terms
-
Anastomosis, Surgical
Animals
Barrett Esophagus/*enzymology/*surgery
Cytoplasm/metabolism
DNA Damage
Disease Models, Animal
Duodenum/*enzymology/surgery
Esophagus/metabolism
Immunohistochemistry
Male
Models, Anatomic
Neoplasms, Experimental/pathology
Nitric Oxide/metabolism
Nitric-Oxide Synthase/*biosynthesis
RNA/metabolism
RNA, Messenger/metabolism
Rats
Rats, Sprague-Dawley
Research Support, Non-U.S. Gov't
Reverse Transcriptase Polymerase Chain Reaction
Time Factors
Figure
Reference
-
1. Schmidt HH, Walter U. NO at work. Cell. 1994. 78:919–925.
Article2. Knowles RG, Moncada S. Nitric oxide synthases in mammals. Biochem J. 1994. 298:249–258.
Article3. Lala PK, Orucevic A. Role of nitric oxide in tumor progression: Lessons from experimental tumors. Cancer Metastasis Rev. 1998. 17:91–106.4. Nathan C, Xie Q. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994. 269:13725–13728.
Article5. Wink DA, Vodovotz Y, Laval J, Laval F, Dewhirst MW, Mitchell JB. The multifaceted roles of nitric oxide in cancer. Carcinogenesis. 1998. 19:711–721.
Article6. Felley-Bosco E. Role of nitric oxide in genotoxicity: implication for carcinogenesis. Cancer Metastasis Rev. 1998. 17:25–37.7. Rachmilewitz D, Karmeli F, Eliakim R, Stalnikowicz R, Ackerman Z, Amir G, Stamler JS. Enhanced gastric nitric oxide synthase activity in duodenal ulcer patients. Gut. 1994. 35:1394–1397.
Article8. Singer II, Kawaka DW, Scott S, Mumford RA, Riehl TE, Stenson WF. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology. 1996. 111:871–885.
Article9. Haswell-Elkins MR, Satarug S, Tsuda M, Mairiang E, Esumi H, Sithithaworn P, Mairiang P, Saitoh M, Yongvanit P, Elkins DB. Liver fluke infection and cholangiocarcinoma: model of endogenous nitric oxide and extragastric nitrosation in human carcinogenesis. Mutat Res. 1994. 305:241–252.
Article10. Wilson KT, Fu S, Ramanujam KS, Meltzer SJ. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett's esophagus and associated adenocarcinomas. Cancer Res. 1998. 58:2929–2934.11. Takahashi M, Fukuda K, Ohata T, Sugimura T, Wakabayashi K. Increased expression of inducible and endothelial constitutive nitric oxide synthases in rat colon tumors induced by azoxymethane. Cancer Res. 1997. 57:1233–1237.12. Goldstein SR, Yang GY, Chen X, Curtis SK, Yang CS. Studies of iron deposits, inducible nitric oxide synthase and nitrotyrosine in a rat model for esophageal adenocarcinoma. Carcinogenesis. 1998. 19:1445–1449.
Article13. Kauer WK, Peters JH, DeMeester TR, Ireland AP, Bremner CG, Hagen JA. Mixed reflux of gastric and duodenal juices is more harmful to the esophagus and gastric juice alone. Ann Surg. 1995. 222:525–533.14. Gillen P, Keeling P, Byrne PJ, Healy M, O'Moore RR, Hennessy TP. Implication of duodenogastric reflux in the pathogenesis of Barrett's esophagus. Br J Surg. 1988. 75:540–543.15. Goldstein SR, Yang GY, Curtis SK, Reuhl KR, Liu BC, Mirvish SS, Newmark HL, Yang CS. Development of esophageal metaplasia and adenocarcinoma in a rat surgical model without the use of a carcinogen. Carcinogenesis. 1997. 18:2265–2270.
Article16. Miwa K, Sahara H, Segawa M, Kinami S, Sato T, Miyazaki I, Hattori T. Reflux of duodenal or gastro-duodenal contents induces esophageal carcinoma in rats. Int J Cancer. 1996. 67:269–274.
Article17. Pera M, Brito MJ, Poulsom R, Riera E, Grande L, Hanby A, Wright NA. Duodenal-content reflux esophagitis induces the development of glandular metaplasia and adenosquamous carcinoma in rats. Carcinogenesis. 2000. 21:1587–1591.
Article18. Jang TJ, Min SK, Bae JD, Jung KH, Lee JI, Kim JR, Ahn WS. Expression of cyclooxygenase-2, microsomal prostaglandin E synthase-1 and EP receptors is increased in rat esophageal squamous cell dysplasia and Barrett's metaplasia induced by duodenal contents reflux. Gut. 2004. 53:27–33.19. Di Rosa M, Ialenti A, Ianaro A, Sautebin L. Interaction between nitric oxide and cyclooxygenase (COX) pathways. Prostaglandins Leukot Essent Fatty Acids. 1996. 54:229–238.20. Clark GW, Smyrk TC, Mirvish SS, Anselmino M, Yamashita Y, Hinder RA, DeMeester TR, Birt DF. Effect of gastroduodenal juice and dietary fat on the development of Barrett's esophagus and esophageal neoplasia: an experimental rat model. Ann Surg Oncol. 1994. 1:252–261.
Article21. Chen X, Yang CS. Esophageal adenocarcinoma: a review and perspectives on the mechanism of carcinogenesis and chemoprevention. Carcinogenesis. 2001. 22:1119–1129.
Article22. Chhatwal VJ, Nagoi SS, Chan ST, Chia YW, Moochhala SM. Aberrant expression of nitric oxide synthase in human polyps, neoplastic colonic mucosa and surrounding peritumoral normal mucosa. Carcinogenesis. 1994. 15:2081–2085.
Article23. Moochhala S, Chhatwal VJ, Chan ST, Ngoi SS, Chia YW, Rauff A. Nitric oxide synthase activity and expression in human colorectal cancer. Carcinogenesis. 1996. 17:1171–1174.
Article24. Scott DJ, Hull MA, Cartwright EJ, Lam WK, Tisbury A, Poulsom R, Markham AF, Bonifer C, Coletta PL. Lack of inducible nitric oxide synthase promotes intestinal tumorigenesis in the ApcMin/+ mouse. Gastroenterology. 2001. 121:889–899.
Article25. Ahn B, Ohshima H. Suppression of intestinal polyposis in ApcMin/+ mice by inhibiting nitric oxide production. Cancer Res. 2001. 61:8357–8360.26. Timoshenko AV, Lala PK, Chakraborty C. PGE2 mediated upregulation of iNOS in murine breast cancer cells through the activation of EP4 receptors. Int J Cancer. 2004. 108:384–389.27. Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci USA. 1993. 90:7240–7244.
Article28. Salvemini D, Seibert K, Masferrer JL, Misko TP, Currie MG, Needleman P. Endogenous nitric oxide enhances prostaglandin production in a model of renal inflammation. J Clin Invest. 1994. 93:1940–1947.
Article29. Buttar NS, Wang KK, Leontovich O, Westcott JY, Pacifico RJ, Anderson MA, Krishnadath KK, Lutzke LS, Burgart LJ. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett's esophagus. Gastroenterology. 2002. 122:1101–1112.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Involvement of Fibronectin in the Migration of Macrophage and Expression of Nitric Oxide Synthase in the BCG induced Inflammatory Sites in Rat Bladder
- Change of Inducible Nitric Oxide Synthase Expression by Ultraviolet B Irradiation on the Skin of a Rat
- Inhibitory Effect of Esculetin on the Inducuble Nitric Oxide Synthase Expression in TNF-stimulated 3T3-L1 Adipocytes
- Expression of Constitutive Nitric Oxide Synthase by Gastrointestinal Epithelial Cells
- Nitric oxide production and inducible nitric oxide synthase expression induced by Porphyromonas gingivalis lipopolysaccharide