J Korean Med Sci.
2011 Sep;26(9):1178-1184. 10.3346/jkms.2011.26.9.1178.
A Novel Biomarker of Coronary Atherosclerosis: Serum DKK1 Concentration Correlates with Coronary Artery Calcification and Atherosclerotic Plaques
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Bundang Hospital, Seongnam, Korea. cheolkim@snu.ac.kr
- 2Department of Laboratory Medicine, Seoul National University College of Medicine, Bundang Hospital, Seongnam, Korea.
- 3Department of Radiology, Seoul National University College of Medicine, Bundang Hospital, Seongnam, Korea.
- KMID: 1779391
- DOI: http://doi.org/10.3346/jkms.2011.26.9.1178
Abstract
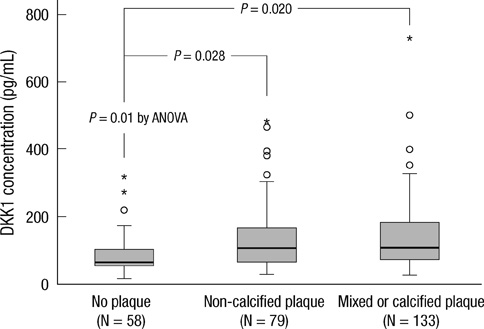
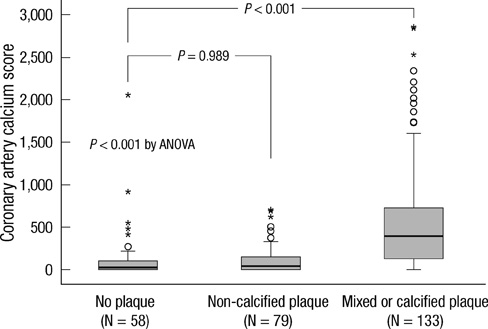
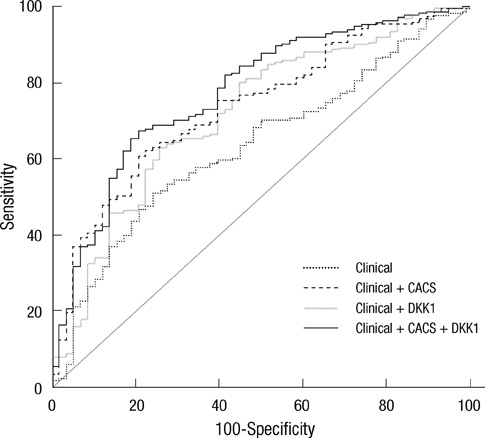
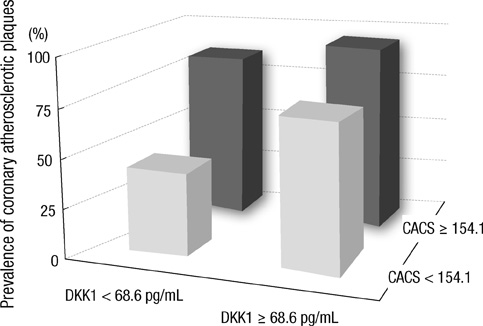
- DKK1 modulates Wnt signaling, which is involved in the atherosclerosis. However, no data exist regarding the usefulness of measuring serum DKK1 concentration in predicting coronary atherosclerosis. A total of 270 consecutive patients (62.8 +/- 11.2 yr; 70% male) were included. A contrast-enhanced 64-slice coronary MDCT was performed to identify the presence of atherosclerotic plaques. Agatston calcium scores (CS) were calculated to quantify the coronary artery calcification (CAC). DKK1 concentrations were measured by enzyme-linked immunosorbent assay. For each subsequent DKK1 quartile, there was a significant increase in CAC (P = 0.004) and the number of segments with coronary atherosclerosis (P < 0.001). In addition, DKK1 concentration was significantly higher in patients with atherosclerotic plaques, regardless of plaque composition (P = 0.01). Multivariate analysis identified DKK1 as an independent risk factor for the presence of coronary atherosclerotic plaque. The adjusted odds ratio for coronary atherosclerotic plaque was 4.88 (95% CI, 1.67 to 14.25) for highest versus lowest quartile of the DKK1 levels. Furthermore, patients with DKK1 concentrations > or = 68.6 pg/mL demonstrated coronary atherosclerotic plaques even when they had low CS. Serum DKK1 concentrations correlate with the coronary atherosclerosis and play an independent role in predicting the presence of coronary atherosclerosis.
Keyword
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Biological Markers/blood
Calcinosis/blood/complications/radiography
Coronary Artery Disease/blood/complications/*diagnosis/radiography
Female
Humans
Intercellular Signaling Peptides and Proteins/*blood
Male
Middle Aged
Odds Ratio
Plaque, Atherosclerotic/blood/*diagnosis
Predictive Value of Tests
Risk Factors
Severity of Illness Index
Tomography, X-Ray Computed
Figure
Reference
-
1. Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O'Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008. 358:1336–1345.2. Rubinshtein R, Halon DA, Gaspar T, Jaffe R, Karkabi B, Flugelman MY, Kogan A, Shapira R, Peled N, Lewis BS. Usefulness of 64-slice cardiac computed tomographic angiography for diagnosing acute coronary syndromes and predicting clinical outcome in emergency department patients with chest pain of uncertain origin. Circulation. 2007. 115:1762–1768.3. Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA. 2004. 291:210–215.4. Einstein AJ, Henzlova MJ, Rajagopalan S. Estimating risk of cancer associated with radiation exposure from 64-slice computed tomography coronary angiography. JAMA. 2007. 298:317–323.5. Bamberg F, Dannemann N, Shapiro MD, Seneviratne SK, Ferencik M, Butler J, Koenig W, Nasir K, Cury RC, Tawakol A, Achenbach S, Brady TJ, Hoffmann U. Association between cardiovascular risk profiles and the presence and extent of different types of coronary atherosclerotic plaque as detected by multidetector computed tomography. Arterioscler Thromb Vasc Biol. 2008. 28:568–574.6. Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis: a look outside the nucleus. Science. 2000. 287:1606–1609.7. Sen M, Ghosh G. Transcriptional outcome of Wnt-Frizzled signal transduction in inflammation: evolving concepts. J Immunol. 2008. 181:4441–4445.8. Christman MA 2nd, Goetz DJ, Dickerson E, McCall KD, Lewis CJ, Benencia F, Silver MJ, Kohn LD, Malgor R. Wnt5a is expressed in murine and human atherosclerotic lesions. Am J Physiol Heart Circ Physiol. 2008. 294:H2864–H2870.9. Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005. 115:1210–1220.10. Kirton JP, Crofts NJ, George SJ, Brennan K, Canfield AE. Wnt/beta-catenin signaling stimulates chondrogenic and inhibits adipogenic differentiation of pericytes: potential relevance to vascular disease? Circ Res. 2007. 101:581–589.11. Ueland T, Otterdal K, Lekva T, Halvorsen B, Gabrielsen A, Sandberg WJ, Paulsson-Berne G, Pedersen TM, Folkersen L, Gullestad L, Oie E, Hansson GK, Aukrust P. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009. 29:1228–1234.12. Pryor DB, Shaw L, McCants CB, Lee KL, Mark DB, Harrell FE Jr, Muhlbaier LH, Califf RM. Value of the history and physical in identifying patients at increased risk for coronary artery disease. Ann Intern Med. 1993. 118:81–90.13. Chun EJ, Lee W, Choi YH, Koo BK, Choi SI, Jae HJ, Kim HC, So YH, Chung JW, Park JH. Effects of nitroglycerin on the diagnostic accuracy of electrocardiogram-gated coronary computed tomography angiography. J Comput Assist Tomogr. 2008. 32:86–92.14. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990. 15:827–832.15. Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975. 51:4 Suppl. 5–40.16. Choi EK, Chun EJ, Choi SI, Chang SA, Choi SH, Lim S, Rivera JJ, Nasir K, Blumenthal RS, Jang HC, Chang HJ. Assessment of subclinical coronary atherosclerosis in asymptomatic patients with type 2 diabetes mellitus with single photon emission computed tomography and coronary computed tomography angiography. Am J Cardiol. 2009. 104:890–896.17. Scholte AJ, Schuijf JD, Kharagjitsingh AV, Jukema JW, Pundziute G, van der Wall EE, Bax JJ. Prevalence of coronary artery disease and plaque morphology assessed by multi-slice computed tomography coronary angiography and calcium scoring in asymptomatic patients with type 2 diabetes. Heart. 2008. 94:290–295.18. Gottlieb I, Miller JM, Arbab-Zadeh A, Dewey M, Clouse ME, Sara L, Niinuma H, Bush DE, Paul N, Vavere AL, Texter J, Brinker J, Lima JA, Rochitte CE. The absence of coronary calcification does not exclude obstructive coronary artery disease or the need for revascularization in patients referred for conventional coronary angiography. J Am Coll Cardiol. 2010. 55:627–634.19. Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006. 25:7469–7481.20. Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998. 391:357–362.21. Panizo S, Cardus A, Encinas M, Parisi E, Valcheva P, Lépez-Ongil S, Coll B, Fernandez E, Valdivielso JM. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ Res. 2009. 104:1041–1048.22. Aicher A, Kollet O, Heeschen C, Liebner S, Urbich C, Ihling C, Orlandi A, Lapidot T, Zeiher AM, Dimmeler S. The Wnt antagonist Dickkopf-1 mobilizes vasculogenic progenitor cells via activation of the bone marrow endosteal stem cell niche. Circ Res. 2008. 103:796–803.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Calcification and Aneurysms of Coronary Artery without Atherosclerosis in Young Adult
- Expression of osteopontin in calcified coronary atherosclerotic plaques
- Assessment of Non-Calcified Coronary Plaques Using 64-Slice Computed Tomography: Comparison With Intravascular Ultrasound
- Statin Therapy with Coronary Plaque Imaging
- Evaluation of Atherosclerotic Plaque in Patients without Coronary Artery Calcification Using Multidetector Row Computed Tomography: A Preliminary Report of 110 patients