J Korean Med Sci.
2011 Jan;26(1):92-99. 10.3346/jkms.2011.26.1.92.
The Role of Endothelin Receptor A during Myelination of Developing Oligodendrocytes
- Affiliations
-
- 1Department of Anatomy, Institute for Brain Research, Chungnam National University School of Medicine, Daejeon, Korea.
- 2Department of Oral Physiology, School of Dentistry, Seoul National University, Seoul, Korea.
- 3Department of Experimental Animal Research, Clinical Research Institute, Seoul National University Hospital, Seoul, Korea. bckang@snu.ac.kr
- 4Graduate School of Immunology, College of Medicine, Seoul National University, Seoul, Korea.
- KMID: 1777982
- DOI: http://doi.org/10.3346/jkms.2011.26.1.92
Abstract
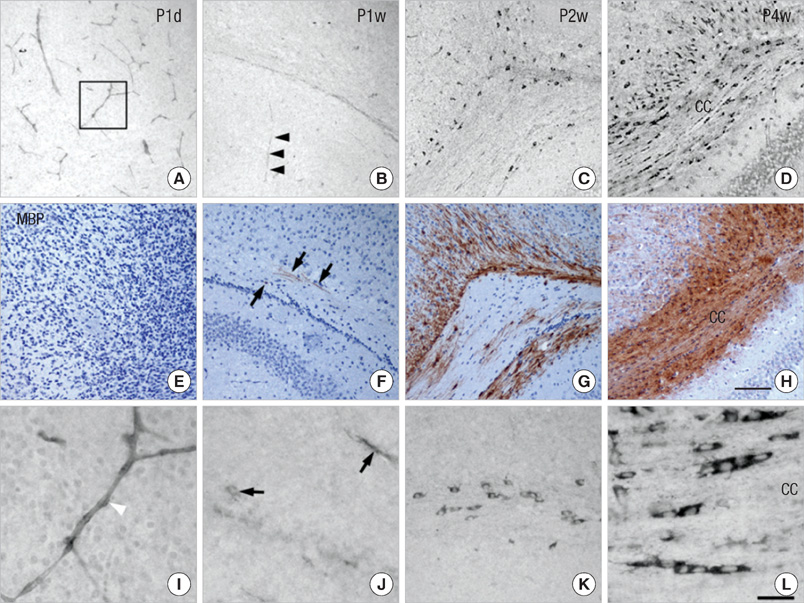
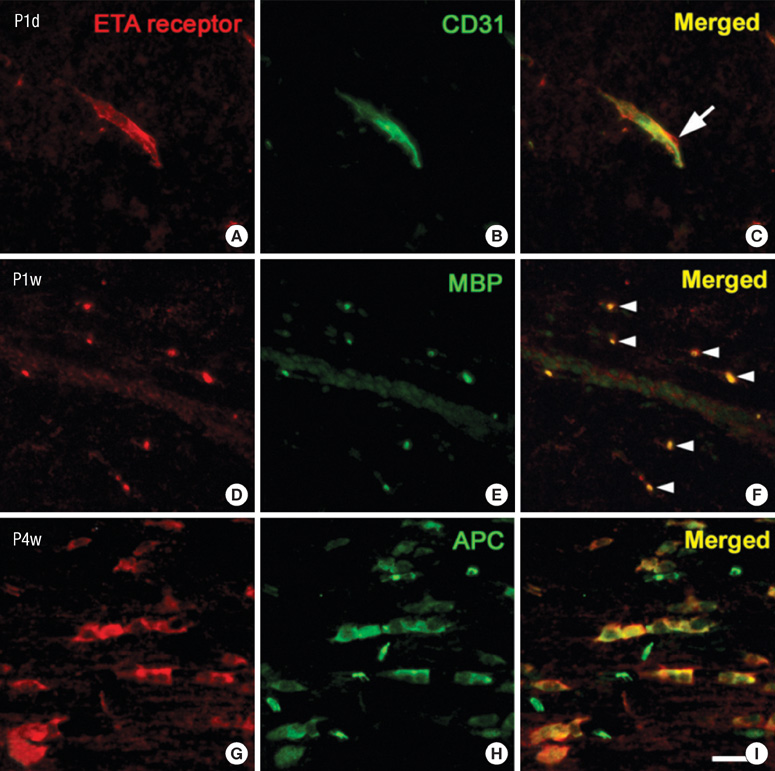
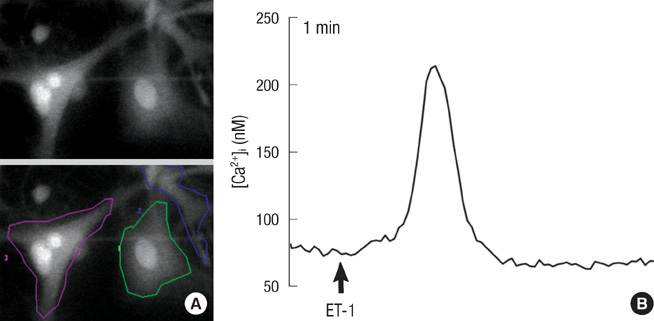
- Endothelin (ET)-1 and its receptors (ETA and ETB receptor) are present in the central nervous system. ET exerts biological effects on gliogenesis and glial cell functions. In order to define a possible mechanism of ETA receptor signaling, the distribution of the ETA receptor in developing oligodendrocytes and the effects of ET-1 on the myelination of oligodendrocytes were examined. ETA receptor immunoreactivity was confined to the perivascular elements of the blood vessels during early postnatal development. However later in development, ETA receptor immunoreactivity was no longer observed in the vessels but became localized to the myelinating oligodendrocytes of the primitive corpus callosum of the white matter, apart from the vessels. ET-1 induced myelin basic protein (MBP) in primary oligodendrocyte precursor cell culture though the ETA receptor and was blocked by an ETA receptor antagonist. In addition, ET-1 evoked the release of Ca2+ which is a central regulator of oligodendrocyte differentiation. Our results provide a link between ET-1 and its ETA receptor and myelination during oligodendrocyte differentiation.
MeSH Terms
Figure
Reference
-
1. Yanagisawa M, Kurihara H, Kimura S, Goto K, Masaki T. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. J Hypertens Suppl. 1988. 6:S188–S191.2. Simonson MS. Endothelins: multifunctional renal peptides. Physiol Rev. 1993. 73:375–411.3. Schinelli S. Pharmacology and physiopathology of the brain endothelin system: an overview. Curr Med Chem. 2006. 13:627–638.4. Li JJ, Wu LH, Cao Q, Yuan Y, Yang L, Guo ZY, Kaur C, Sivakumar V, Ling EA, Wu CY. Endothelins-1/3 and endothelin-A/B receptors expressing glial cells with special reference to activated microglia in experimentally induced cerebral ischemia in the adult rats. Neuroscience. 2010. 167:665–677.5. Thomas AJ, Erickson CA. The making of a melanocyte: the specification of melanoblasts from the neural crest. Pigment Cell Melanoma Res. 2008. 21:598–610.6. Yoo KH, Yim HE, Jang GY, Bae IS, Choi BM, Hong YS, Lee JW. Endothelin A receptor blockade influences apoptosis and cellular proliferation in the developing rat kidney. J Korean Med Sci. 2009. 24:138–145.7. Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990. 348:730–732.8. Avedanian L, Riopel J, Bkaily G, Nader M, D'Orleans-Juste P, Jacques D. ETA receptors are present in human aortic vascular endothelial cells and modulate intracellular calcium. Can J Physiol Pharmacol. 2010. 88:817–829.9. Grantcharova E, Reusch HP, Beyermann M, Rosenthal W, Oksche A. Endothelin A and endothelin B receptors differ in their ability to stimulate ERK1/2 activation. Exp Biol Med (Maywood). 2006. 231:757–760.10. Bradl M, Lassmann H. Oligodendrocytes: biology and pathology. Acta Neuropathol. 2010. 119:37–53.11. Gadea A, Aguirre A, Haydar TF, Gallo V. Endothelin-1 regulates oligodendrocyte development. J Neurosci. 2009. 29:10047–10062.12. Shin T, Kang B, Tanuma N, Matsumoto Y, Wie M, Ahn M, Kang J. Intrathecal administration of endothelin-1 receptor antagonist ameliorates autoimmune encephalomyelitis in Lewis rats. Neuroreport. 2001. 12:1465–1468.13. Kim DW, Chang JH, Park SW, Jeon GS, Seo JH, Cho SS. Activated cyclic AMP-response element binding protein (CREB) is expressed in a myelin-associated protein in chick. Neurochem Res. 2005. 30:1133–1137.14. Chen Y, Balasubramaniyan V, Peng J, Hurlock EC, Tallquist M, Li J, Lu QR. Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat Protoc. 2007. 2:1044–1051.15. Cohen RI, Almazan G. Rat oligodendrocytes express muscarinic receptors coupled to phosphoinositide hydrolysis and adenylyl cyclase. Eur J Neurosci. 1994. 6:1213–1224.16. Radhakrishna M, Almazan G. Protein kinases mediate basic fibroblast growth factor's stimulation of proliferation and c-fos induction in oligodendrocyte progenitors. Brain Res Mol Brain Res. 1994. 24:118–128.17. Agresti C, D'Urso D, Levi G. Reversible inhibitory effects of interferon-gamma and tumour necrosis factor-alpha on oligodendroglial lineage cell proliferation and differentiation in vitro. Eur J Neurosci. 1996. 8:1106–1116.18. Paez PM, Fulton D, Colwell CS, Campagnoni AT. Voltage-operated Ca(2+) and Na(+) channels in the oligodendrocyte lineage. J Neurosci Res. 2009. 87:3259–3266.19. Nambi P, Pullen M, Feuerstein G. Identification of endothelin receptors in various regions of rat brain. Neuropeptides. 1990. 16:195–199.20. Calza L, Fernandez M, Giuliani A, Aloe L, Giardino L. Thyroid hormone activates oligodendrocyte precursors and increases a myelin-forming protein and NGF content in the spinal cord during experimental allergic encephalomyelitis. Proc Natl Acad Sci USA. 2002. 99:3258–3263.21. Ono K, Bansal R, Payne J, Rutishauser U, Miller RH. Early development and dispersal of oligodendrocyte precursors in the embryonic chick spinal cord. Development. 1995. 121:1743–1754.22. Kim DW, Park SW, Jeon GS, Seo JH, Golden JA, Cho SS. The multiple dorsoventral origins and migratory pathway of tectal oligodendrocytes in the developing chick. Brain Res. 2006. 1076:16–24.23. Peters CM, Rogers SD, Pomonis JD, Egnaczyk GF, Keyser CP, Schmidt JA, Ghilardi JR, Maggio JE, Mantyh PW. Endothelin receptor expression in the normal and injured spinal cord: potential involvement in injury-induced ischemia and gliosis. Exp Neurol. 2003. 180:1–13.24. Pomonis JD, Rogers SD, Peters CM, Ghilardi JR, Mantyh PW. Expression and localization of endothelin receptors: implications for the involvement of peripheral glia in nociception. J Neurosci. 2001. 21:999–1006.25. Butt AM, Pugh M, Hubbard P, James G. Functions of optic nerve glia: axoglial signalling in physiology and pathology. Eye (Lond). 2004. 18:1110–1121.26. Butt AM. Neurotransmitter-mediated calcium signalling in oligodendrocyte physiology and pathology. Glia. 2006. 54:666–675.27. Soliven B. Calcium signalling in cells of oligodendroglial lineage. Microsc Res Tech. 2001. 52:672–679.28. Kawanabe Y, Nauli SM. Involvement of extracellular Ca2+ influx through voltage-independent Ca2+ channels in endothelin-1 function. Cell Signal. 2005. 17:911–916.29. Prineas JW, Kwon EE, Cho ES, Sharer LR, Barnett MH, Oleszak EL, Hoffman B, Morgan BP. Immunopathology of secondary-progressive multiple sclerosis. Ann Neurol. 2001. 50:646–657.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunochemical Study on the Changes of Carbonic anhydrase-II and Iron-binding Proteins in the Demyelinationand and Remyelination model Mouse induced with Cuprizone
- Pathophysiological Role of Endothelin-1 and Clinical Usefulness of Endothelin Antagonists
- Carbonic anhydrase II immunostaining in the cerebellum of postnatal mice
- On the Origin of Oligodendrocytes
- Beyond Vasodilator: Anti-Apoptotic Effect of Endothelin Receptor Antagonist