Yonsei Med J.
2011 May;52(3):522-526. 10.3349/ymj.2011.52.3.522.
Effect of Pertussis Toxin and Herbimycin A on Proteinase-Activated Receptor 2-Mediated Cyclooxygenase 2 Expression in Helicobacter pylori-Infected Gastric Epithelial AGS Cells
- Affiliations
-
- 1Department of Pharmacology, Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Physiology, Tissue Injury Defense Research Center, College of Medicine, Ewha Womans University, Seoul, Korea.
- 3Institute of Food and Nutritional Science, College of Human Ecology, Yonsei University, Seoul, Korea.
- 4Department of Food and Nutrition, Brain Korea 21 Project, College of Human Ecology, Yonsei University, Seoul, Korea. kim626@yonsei.ac.kr
- KMID: 1777026
- DOI: http://doi.org/10.3349/ymj.2011.52.3.522
Abstract
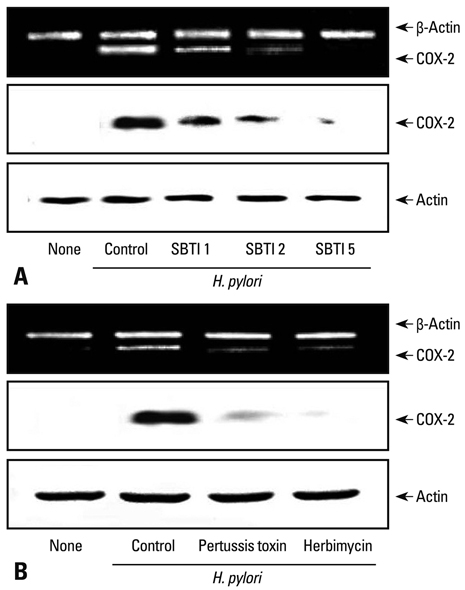
- Helicobacter pylori (H. pylori) is an important risk factor for chronic gastritis, peptic ulcer, and gastric cancer. Proteinase-activated receptor 2 (PAR2), subgroup of G-protein coupled receptor family, is highly expressed in gastric cancer, and chronic expression of cyclooxygenase-2 (COX-2) plays an important role in H. pylori-associated gastric carcinogenesis and inflammation. We previously demonstrated that H. pylori induced the expression of PAR2 and COX-2 in gastric epithelial cells. Present study aims to investigate whether COX-2 expression induced by H. pylori in Korean isolates is mediated by PAR2 via activation of Gi protein and Src kinase in gastric epithelial AGS cells. Results showed that H. pylori-induced COX-2 expression was inhibited in the cells transfected with antisense oligonucleotide for PAR2 or treated with Gi protein blocker pertussis toxin, Src kinase inhibitor herbimycin A and soybean trypsin inbitor, indicating that COX-2 expression is mediated by PAR2 through activation of Gi protein and Src kinase in gastric epithelial cells infected with H. pylori in Korean isolates. Thus, targeting the activation of PAR2 may be beneficial for prevention or treatment of gastric inflammation and carcinogenesis associated with H. pylori infection.
MeSH Terms
-
Benzoquinones/*pharmacology
Cell Line, Tumor
Cyclooxygenase 2/genetics/*metabolism
Epithelial Cells/enzymology/metabolism/microbiology
GTP-Binding Protein alpha Subunits, Gi-Go/metabolism
Gastric Mucosa/enzymology/metabolism/*microbiology
*Helicobacter pylori
Humans
Lactams, Macrocyclic/*pharmacology
Oligonucleotides, Antisense
Pertussis Toxin/*pharmacology
RNA, Messenger/metabolism
Receptor, PAR-2/*physiology
src-Family Kinases/metabolism
Figure
Reference
-
1. Tatsuguchi A, Sakamoto C, Wada K, Akamatsu T, Tsukui T, Miyake K, et al. Localisation of cyclooxygenase 1 and cyclooxygenase 2 in Helicobacter pylori related gastritis and gastric ulcer tissues in humans. Gut. 2000. 46:782–789.
Article2. McCarthy CJ, Crofford LJ, Greenson J, Scheiman JM. Cyclooxygenase-2 expression in gastric antral mucosa before and after eradication of Helicobacter pylori infection. Am J Gastroenterol. 1999. 94:1218–1223.
Article3. Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W, et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci U S A. 1994. 91:12013–12017.
Article4. Boolbol SK, Dannenberg AJ, Chadburn A, Martucci C, Guo XJ, Ramonetti JT, et al. Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res. 1996. 56:2556–2560.5. Lim JW, Kim H, Kim KH. Nuclear factor-kappaB regulates cyclooxygenase-2 expression and cell proliferation in human gastric cancer cells. Lab Invest. 2001. 81:349–360.
Article6. Kim H, Lim JW, Kim KH. Helicobacter pylori-induced expression of interleukin-8 and cyclooxygenase-2 in AGS gastric epithelial cells: mediation by nuclear factor-kappaB. Scand J Gastroenterol. 2001. 36:706–716.
Article7. Lim JW, Kim H, Kim KH. Cell adhesion-related gene expression by Helicobacter pylori in gastric epithelial AGS cells. Int J Biochem Cell Biol. 2003. 35:1284–1296.
Article8. Yada K, Shibata K, Matsumoto T, Ohta M, Yokoyama S, Kitano S. Protease-activated receptor-2 regulates cell proliferation and enhances cyclooxygenase-2 mRNA expression in human pancreatic cancer cells. J Surg Oncol. 2005. 89:79–85.
Article9. Seo JH, Lim JW, Yoon JH, Kim H. Proteinase-activated receptor-2 mediates the expression of integrin alpha5 and beta1 in helicobacter pylori-infected gastric epithelial AGS cells. Digestion. 2009. 80:40–49.
Article10. Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci U S A. 1994. 91:9208–9212.
Article11. Xu WF, Andersen H, Whitmore TE, Presnell SR, Yee DP, Ching A, et al. Cloning and characterization of human protease-activated receptor 4. Proc Natl Acad Sci U S A. 1998. 95:6642–6646.
Article12. Hou L, Kapas S, Cruchley AT, Macey MG, Harriott P, Chinni C, et al. Immunolocalization of protease-activated receptor-2 in skin: receptor activation stimulates interleukin-8 secretion by keratinocytes in vitro. Immunology. 1998. 94:356–362.
Article13. Coughlin SR, Camerer E. PARticipation in inflammation. J Clin Invest. 2003. 111:25–27.
Article14. Hamilton JR, Frauman AG, Cocks TM. Increased expression of protease-activated receptor-2 (PAR2) and PAR4 in human coronary artery by inflammatory stimuli unveils endothelium-dependent relaxations to PAR2 and PAR4 agonists. Circ Res. 2001. 89:92–98.
Article15. Kamath L, Meydani A, Foss F, Kuliopulos A. Signaling from protease-activated receptor-1 inhibits migration and invasion of breast cancer cells. Cancer Res. 2001. 61:5933–5940.16. Labigne A, de Reuse H. Determinants of Helicobacter pylori pathogenicity. Infect Agents Dis. 1996. 5:191–202.17. Blaser MJ, Perez-Perez GI, Kleanthous H, Cover TL, Peek RM, Chyou PH, et al. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995. 55:2111–2115.18. Kuipers EJ, Pérez-Pérez GI, Meuwissen SG, Blaser MJ. Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst. 1995. 87:1777–1780.
Article19. Bach S, Makristathis A, Rotter AM, Hirschl M. Gene expression profiling in AGS cells stimulated with Helicobacter pylori isogenic strains (cagA positive or cagA negative). Infect Immun. 2002. 70:988–992.
Article20. Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999. 37:2274–2279.
Article21. Moss J, Bruni P, Hsia JA, Tsai SC, Watkins PA, Halpern JL, et al. Pertussis toxin-catalyzed ADP-ribosylation: effects on the coupling of inhibitory receptors to the adenylate cyclase system. J Recept Res. 1984. 4:459–474.
Article22. Uehara Y, Fukazawa H, Murakami Y, Mizuno S. Irreversible inhibition of v-src tyrosine kinase activity by herbimycin A and its abrogation by sulfhydryl compounds. Biochem Biophys Res Commun. 1989. 163:803–809.
Article23. Seo JH, Lim JW, Kim H, Kim KH. Helicobacter pylori in a Korean isolate activates mitogen-activated protein kinases, AP-1, and NF-kappaB and induces chemokine expression in gastric epithelial AGS cells. Lab Invest. 2004. 84:49–62.
Article24. Cho SO, Lim JW, Kim KH, Kim H. Involvement of Ras and AP-1 in Helicobacter pylori-induced expression of COX-2 and iNOS in gastric epithelial AGS cells. Dig Dis Sci. 2010. 55:988–996.
Article25. Seo JH, Kim KH, Kim H. Role of proteinase-activated receptor-2 on cyclooxygenase-2 expression in H. pylori-infected gastric epithelial cells. Ann N Y Acad Sci. 2007. 1096:29–36.
Article26. LaMorte VJ, Harootunian AT, Spiegel AM, Tsien RY, Feramisco JR. Mediation of growth factor induced DNA synthesis and calcium mobilization by Gq and Gi2. J Cell Biol. 1993. 121:91–99.
Article27. Della Rocca GJ, Maudsley S, Daaka Y, Lefkowitz RJ, Luttrell LM. Pleiotropic coupling of G protein-coupled receptors to the mitogen-activated protein kinase cascade. Role of focal adhesions and receptor tyrosine kinases. J Biol Chem. 1999. 274:13978–13984.
Article28. Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol. 1999. 11:177–183.
Article29. Seo M, Nam HJ, Kim SY, Juhnn YS. Inhibitory heterotrimeric GTP-binding proteins inhibit hydrogen peroxide-induced apoptosis by up-regulation of Bcl-2 via NF-kappaB in H1299 human lung cancer cells. Biochem Biophys Res Commun. 2009. 381:153–158.
Article30. Goon Goh F, Sloss CM, Cunningham MR, Nilsson M, Cadalbert L, Plevin R. G-protein-dependent and -independent pathways regulate proteinase-activated receptor-2 mediated p65 NFkappaB serine 536 phosphorylation in human keratinocytes. Cell Signal. 2008. 20:1267–1274.
Article31. Manukyan M, Nalbant P, Luxen S, Hahn KM, Knaus UG. RhoA GTPase activation by TLR2 and TLR3 ligands: connecting via Src to NF-kappa B. J Immunol. 2009. 182:3522–3529.
Article32. Hsieh HL, Tung WH, Wu CY, Wang HH, Lin CC, Wang TS, et al. Thrombin induces EGF receptor expression and cell proliferation via a PKC(delta)/c-Src-dependent pathway in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2009. 29:1594–1601.
Article33. Li G, Han C, Xu L, Lim K, Isse K, Wu T. Cyclooxygenase-2 prevents fas-induced liver injury through up-regulation of epidermal growth factor receptor. Hepatology. 2009. 50:834–843.
Article34. Lee TS, Jeon YT, Kim JW, Park NH, Kang SB, Lee HP, et al. Lack of association of the cyclooxygenase-2 and inducible nitric oxide synthase gene polymorphism with risk of cervical cancer in Korean population. Ann N Y Acad Sci. 2007. 1095:134–142.
Article35. Noyan T, Guducuoglu H, Ilhan M. A study of oxidative stress parameters in anti-helicobacter pylorus immunoglobulin G positive and negative gastric cancer patients. Yonsei Med J. 2009. 50:677–682.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Rosiglitazone on the Cell Proliferation and the Expressions of p27 and Skp2 in Helicobacter pylori Infected Human Gastric Epithelial Cells
- Cyclooxygenase-2 expression and apoptosis in Helicobacter pylori-infected human gastric epithelial cells
- Caspase-3 Activation Leads to Apoptosis of Human Gastric Epithelial Cells Infected with Helicobacter pylori
- Jak1/Stat3 Is an Upstream Signaling of NF-kappaB Activation in Helicobacter pylori-Induced IL-8 Production in Gastric Epithelial AGS Cells
- Astaxanthin Prevents Decreases in Superoxide Dismutase 2 Level and Superoxide Dismutase Activity in Helicobacter pylori-infected Gastric Epithelial Cells