Korean J Gastroenterol.
2010 Apr;55(4):225-231. 10.4166/kjg.2010.55.4.225.
The Effect of Rosiglitazone on the Cell Proliferation and the Expressions of p27 and Skp2 in Helicobacter pylori Infected Human Gastric Epithelial Cells
- Affiliations
-
- 1Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea. yscho@catholic.ac.kr
- 2Department of Pathology, The Catholic University of Korea College of Medicine, Seoul, Korea.
- KMID: 1792772
- DOI: http://doi.org/10.4166/kjg.2010.55.4.225
Abstract
-
BACKGROUND/AIMS: Ligands for peroxisome proliferator-activated receptor gamma (PPAR gamma), a member of the ligand-activated nuclear receptor superfamily, exhibit anti-tumoral effects and are associated with de novo synthesis of proteins involved in regulating the cell cycle and cell survival/death. Helicobacter pylori (H. pylori) is an etiologic agent for gastric adenocarcinoma, and raises the cell turnover of gastric epithelium. The aim of this study was to investigate the effect of PPAR gamma ligand rosiglitazone on the cell proliferation and the expressions of p27 and Skp2 protein in H. pylori infected gastric epithelial cells.
METHODS
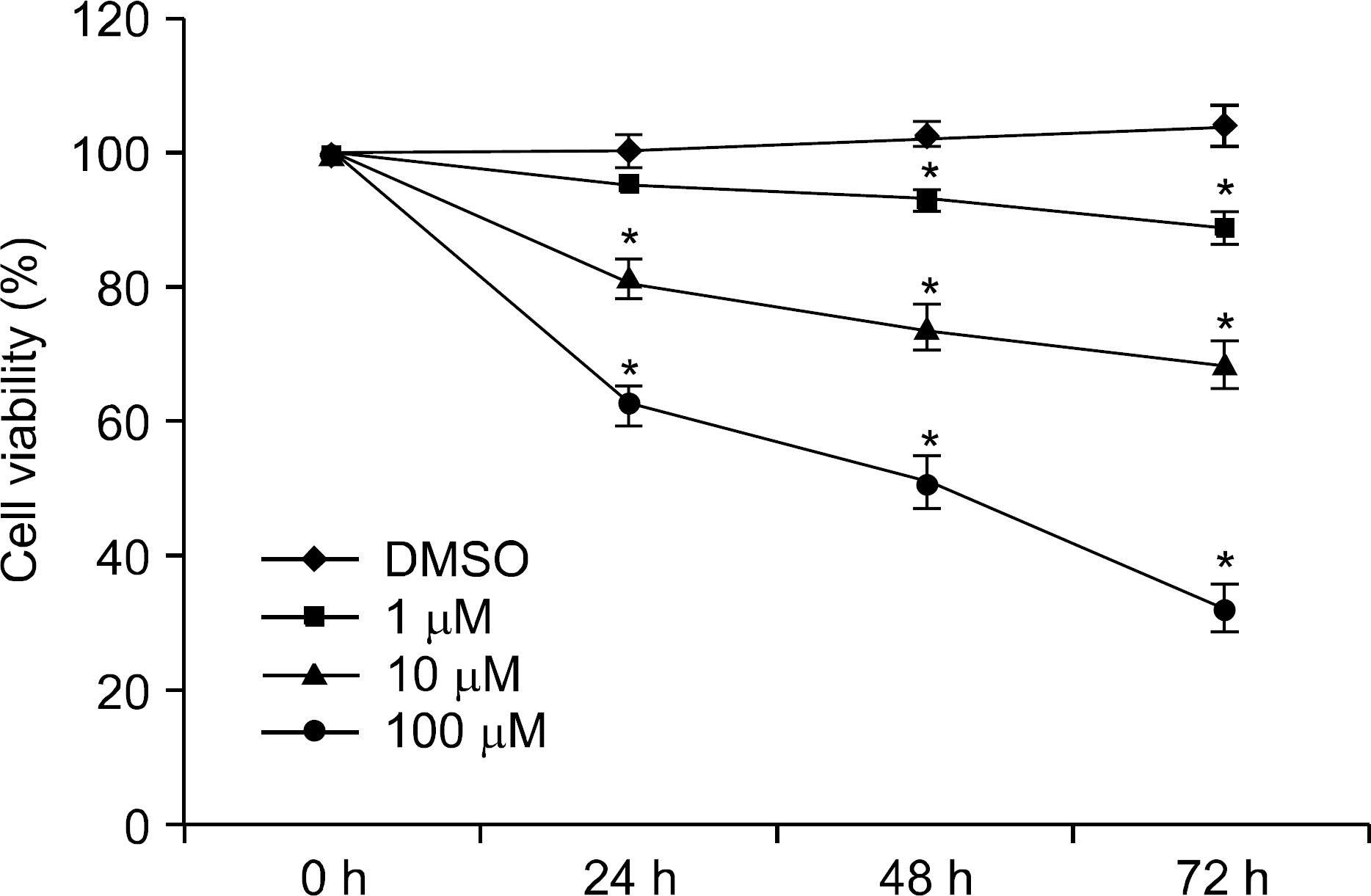
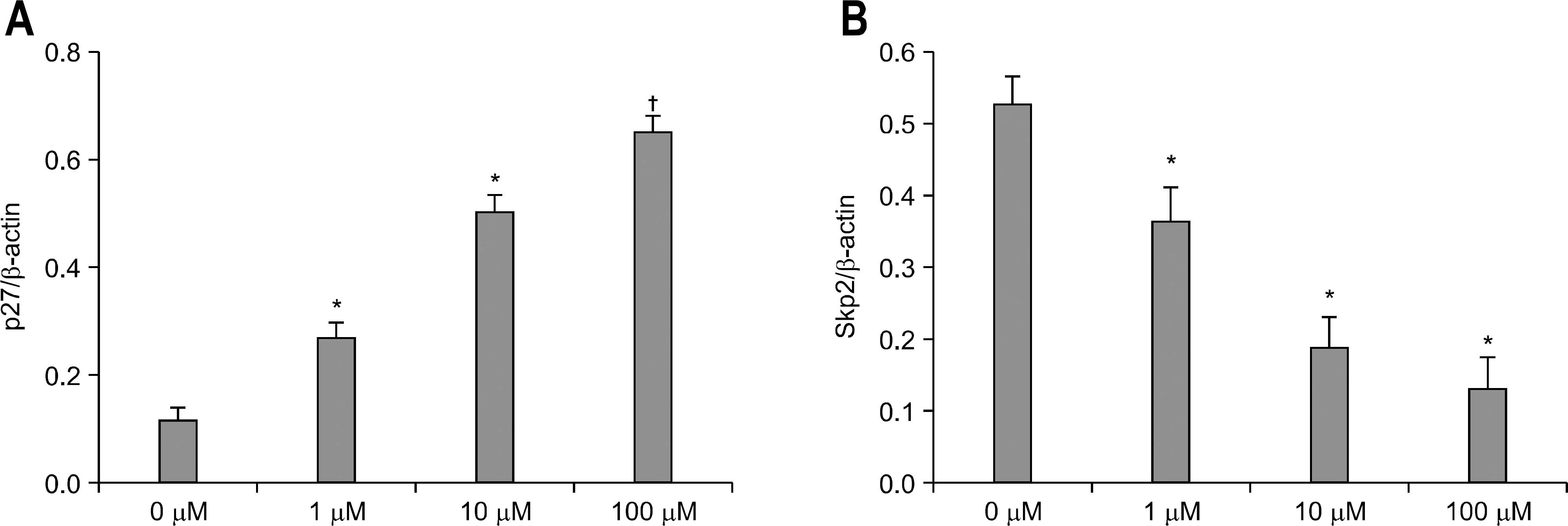
We examined the expression of PPAR gamma by Western blot in H. pylori infected AGS human gastric epithelial cells. The effect of rosiglitazone on the survival of H. pylori infected AGS cells was assessed by cell viability assay. After the treatment of rosiglitazone in H. pylori infected AGS cells, the expressions of p27 and Skp2 were assessed by Western blot.
RESULTS
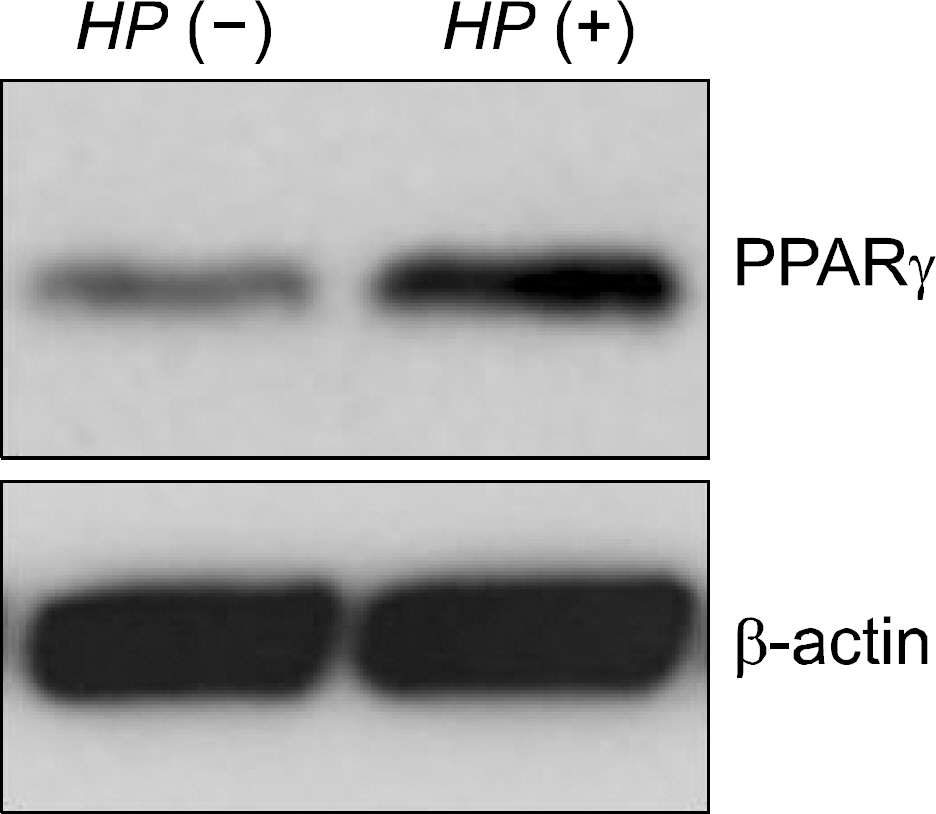
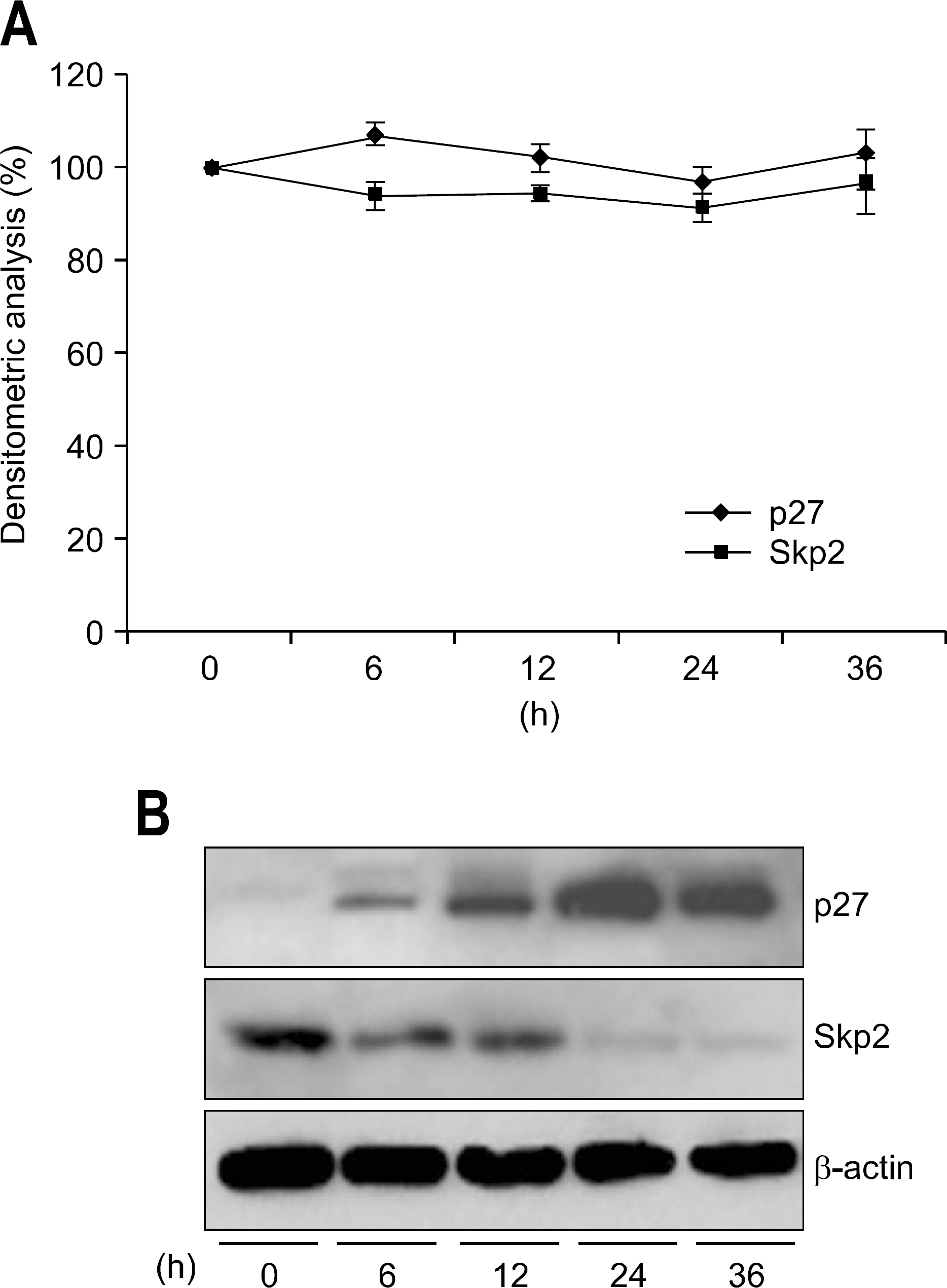
The expression of PPAR gamma protein was increased in H. pylori infected AGS cells. Cell growth was inhibited and decreased in dose- and time- dependent manner in H. pylori infected AGS cells treated with rosiglitazone. A decrease in Skp2 expression and a reciprocal increase in p27 expression were found in dose- and time-dependent manner in H. pylori infected AGS cells treated with rosiglitazone.
CONCLUSIONS
Rosiglitazone inhibited the growth of H. pylori infected AGS cells. Rosiglitazone attenuated Skp2 expression, thereby promoting p27 accumulation in H. pylori infected human gastric epithelial cells. Further studies will be needed to find the effects of accumulation on cell turnover in H. pylori infection and the role in the H. pylori-associated gastric carcinogenesis.
Keyword
MeSH Terms
-
Anti-Bacterial Agents/*pharmacology
Cell Line
Cell Proliferation
Cyclin-Dependent Kinase Inhibitor p27/*metabolism
Epithelial Cells/metabolism/*microbiology
Gastric Mucosa/cytology/metabolism/*microbiology
*Helicobacter pylori
Humans
PPAR gamma/antagonists &inhibitors/metabolism
S-Phase Kinase-Associated Proteins/*metabolism
Thiazolidinediones/*pharmacology
Figure
Reference
-
1. Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995; 83:835–839.
Article2. Tontonoz P, Singer S, Forman BM, et al. Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor gammaand the retinoid X receptor. Proc Natl Acad Sci USA. 1997; 94:237–241.3. Elstner E, Mü ller C, Koshizuka K, et al. Ligands for peroxisome proliferator-activated receptor gamma and the retinoid acid receptor inhibit growth and induce apotosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci USA. 1998; 95:8806–8811.4. Kubota T, Koshizuka K, Williamson EA, et al. Ligands for peroxisome proliferator-activated receptor gamma (troglitazone) has potent antitumor effect against human prostate cancer in vitro and in vivo. Cancer Res. 1998; 58:3344–3352.5. Sarraf P, Mueller E, Jones D, et al. Differentiation and re-versal of malignant changes in colon cancer through PPAR-gamma. Nat Med. 1998; 4:1046–1052.6. Leung WK, Bai AH, Chan VY, et al. Effect of peroxisome proliferator-activated receptor gamma on growth and gene expression profiles of gastric cancer cells. Gut. 2004; 53:331–338.7. Koga H, Sakisaka S, Harada M, et al. Involvement of p21 WAF1/Cip1, p27 Kip1, and p18 INK4c in troglitazone-induced cell cycle arrest in human hepatoma cell lines. Hepatology. 2001; 33:1087–1097.8. Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator- activated receptors and cancers: complex stories. Nat Rev Cancer. 2004; 4:61–70.9. Peek RM Jr, Moss SF, Tham KT, et al. Helicobacter pylori cagA+ strains and dissociation of gastric epithelial proliferation from apoptosis. J Natl Cancer Inst. 1997; 89:863–868.10. Eguchi H, Herschenhous N, Kuzushita N, Moss SF. Helicobacter pylori increases proteasome-mediated degradation of p27 kip1 in gastric epithelial cells. Cancer Res. 2003; 63:4739–4746.11. Shirin H, Sordillo EM, Kolevska TK, et al. Chronic Helicobacter pylori infection induces an apoptosis resistant phenotype associated with decreased expression of p27 kip1. Infect Immun. 2000; 68:5321–5328.12. Yu J, Leung WK, Ng EK, et al. Effect of Helicobacter pylori eradication on expression of cyclin D2 and p27 in gastric intestinal metaplasia. Aliment Pharmacol Ther. 2001; 15:1505–1511.13. Kim SS, Meitner P, Konkin TA, Cho YS, Resnick MB, Moss SF. Altered expression of Skp2, c-Myc and p27 proteins but not mRNA after H. pylori eradication in chronic gastritis. Mod Pathol. 2006; 19:49–58.14. Philipp-Staheli J, Payne SR, Kemp CJ. p27 Kip1: regulation and function of a haploinsufficient tumor suppressor and its misregulation in cancer. Exp Cell Res. 2001; 264:148–168.15. Mori M, Mimori K, Shiraishi T, et al. p27 expression and gastric carcinioma. Nat Med. 1997; 3:593.16. Ohtani M, Isozaki H, Fujii K, et al. Impact of the expression of cyclindependent kinase inhibitor p27 Kip1 and apoptosis in tumor cells on the overall survival of patients with non-early stage gastric carcinoma. Cancer. 1999; 85:1711–1718.17. Martin E, Cacheux V, Cavé H, Lapierre JM, Le Paslier D, Grandchamp B. Localization of the CDKN4/p27 Kip1 gene to human chromosome 12p12.3. Hum Genet. 1995; 96:668–670.18. Wagner S, Beil W, Westermann J, et al. Regulation of gastric epithelial cell growth by Helicobacter pylori: offdence for a major role of apoptosis. Gastroenterology. 1997; 113:1836–1847.19. Son SH, Kim HK, Ji JS, et al. Expression of peroxisome pro-liferator-activated receptor (PPAR) gamma in Helicobacter pylori-infected gastric epithelium. Korean J Gastroenterol. 2007; 49:72–78.20. Keates S, Keates AC, Warny M, Peek RM Jr, Murray PG, Kelly CP. Differential activation of mitogen-activated protein kinases in AGS gastric epithelial cells by cag+ and cag− Helicobacter pylori. J Immunol. 1999; 163:5552–5559.21. Hirata Y, Maeda S, Mitsuno Y, et al. Helicobacter pylori ac-tivates the cyclin D1 gene through mitogen-activated protein kinase pathway in gastric cancer cells. Infect Immun. 2001; 69:3965–3971.22. Shirin H, Sordillo EM, Oh SH, et al. Helicobacter pylori in-hibits the G1 to S transition in AGS gastric epithelial cells. Cancer Res. 1999; 59:2277–2281.23. Loda M, Cukor B, Tam SW, et al. Increased proteasome-dependent degradation of the cyclindependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997; 3:231–234.
Article24. Eguchi H, Carpentier S, Kim SS, Moss SF. p27 kip1 regulates the apoptotic response of gastric epithelial cells to Helicobacter pylori. Gut. 2004; 53:797–804.25. von der Lehr N, Johansson S, Wu S, et al. The F-box protein Skp2 participates in c-Myc proteosomal degradation and acts as a cofactor for c-Myc-regulated transcription. Mol Cell. 2003; 11:1189–1200.
Article26. Kamura T, Hara T, Kotoshiba S, et al. Degradation of p57 Kip2 mediated by SCFSkp2-dependent ubiquitylation. Proc Natl Acad Sci USA. 2003; 100:10231–10236.27. Liang M, Liang YY, Wrighton K, et al. Ubiquitination and proteolysis of cancer-derived Smad4 mutants by SCF Skp2. Mol Cell Biol. 2004; 24:7524–7537.28. Marti A, Wirbelauer C, Scheffner M, Krek W. Interaction between ubiquitin-protein ligase SCF SKP2 and E2F-1 underlies the regulation of E2F-1 degradation. Nat Cell Biol. 1999; 1:14–19.29. Masuda TA, Inoue H, Sonoda H, et al. Clinical and bio-logical significance of S-phase kinase-associated protein 2 (Skp2) gene expression in gastric carcinoma: modulation of malignant phenotype by Skp2 overexpression, possibly via p27 proteolysis. Cancer Res. 2002; 62:3819–3825.30. Ma XM, Liu Y, Guo JW, Liu JH, Zuo LF. Relation of over-expression of S phase kinase-associated protein 2 with reduced expression of p27 and PTEN in human gastric carcinoma. World J Gastroenterol. 2005; 11:6716–6721.
Article31. Gupta RA, Polk DB, Krishna U, et al. Activation of peroxisome proliferator-activated receptor gamma suppresses nuclear factor kappa B-mediated apoptosis induced by Helicobacter pylori in gastric epithelial cells. J Biol Chem. 2001; 276:31059–31066.32. Peek RM Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002; 2:28–37.33. Koga H, Harada M, Ohtsubo M, et al. Troglitazone induces p27 kip1-associated cell-cycle arrest through down-regulating Skp2 in human hepatoma cells. Hepatology. 2003; 37:1086–1096.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Caspase-3 Activation Leads to Apoptosis of Human Gastric Epithelial Cells Infected with Helicobacter pylori
- Effect of Helicobacter pylori eradication on proliferation and apoptosis of gastric epithelial cells
- Cyclooxygenase-2 expression and apoptosis in Helicobacter pylori-infected human gastric epithelial cells
- MicroRNA-186 targets SKP2 to induce p27(Kip1)-mediated pituitary tumor cell cycle deregulation and modulate cell proliferation
- Proinflammatory Cytokine Gene Expression in Human Gastric Epithelial Cells Induced by Increased Motility of H. pylori