Yonsei Med J.
2013 Jan;54(1):246-252. 10.3349/ymj.2013.54.1.246.
The Genetically Modified Polysialylated Form of Neural Cell Adhesion Molecule-Positive Cells for Potential Treatment of X-Linked Adrenoleukodystrophy
- Affiliations
-
- 1Department of Physiology, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Laboratory Medicine and Cell Therapy Center, Yonsei University College of Medicine, Seoul, Korea.
- 3Division of Pediatric Neurology, Department of Pediatrics, Yonsei University College of Medicine, Seoul, Korea. hipo0207@yuhs.ac
- KMID: 1776946
- DOI: http://doi.org/10.3349/ymj.2013.54.1.246
Abstract
- PURPOSE
Cell transplantation of myelin-producing exogenous cells is being extensively explored as a means of remyelinating axons in X-linked adrenoleukodystrophy. We determined whether 3,3',5-Triiodo-L-thyronine (T3) overexpresses the ABCD2 gene in the polysialylated (PSA) form of neural cell adhesion molecule (NCAM)-positive cells and promotes cell proliferation and favors oligodendrocyte lineage differentiation.
MATERIALS AND METHODS
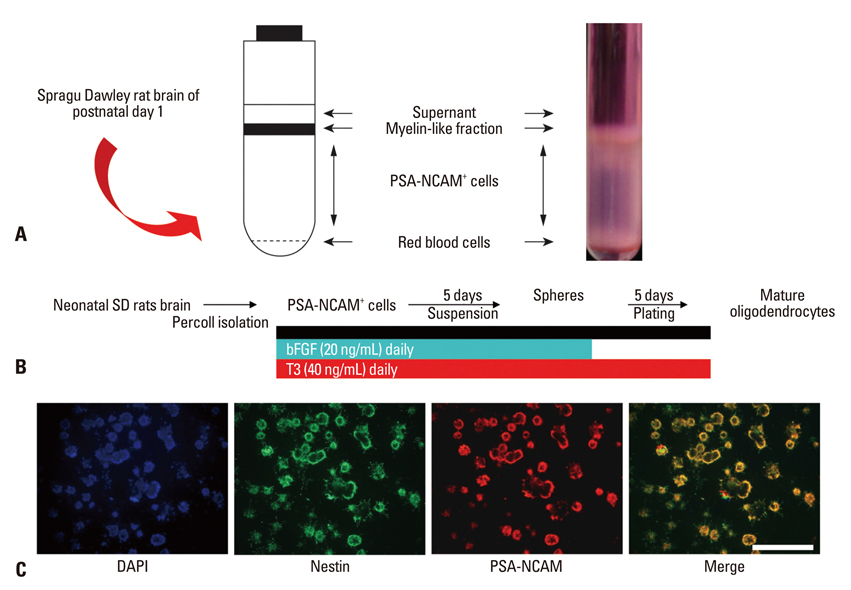
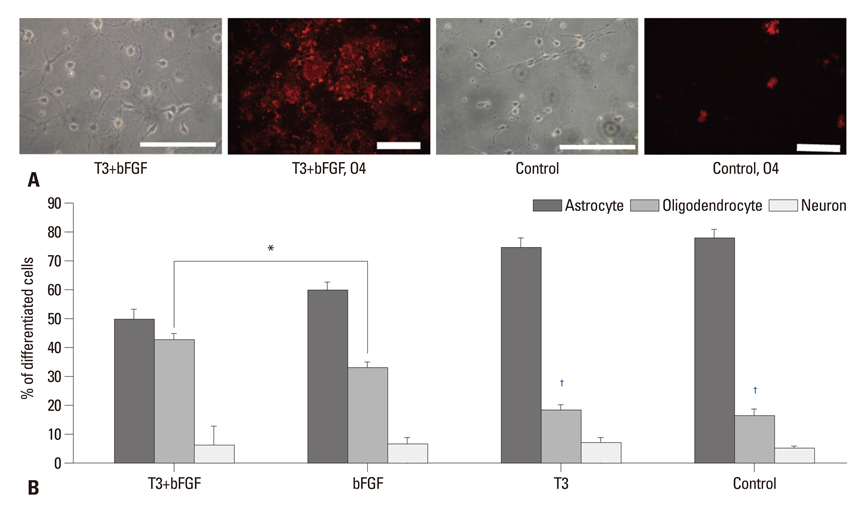
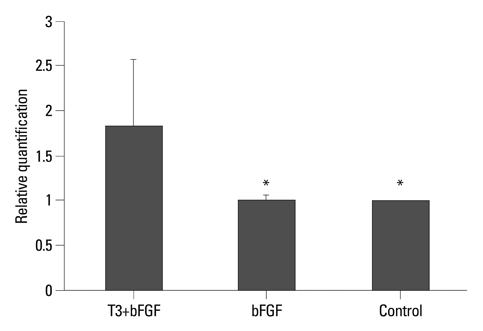
PSA-NCAM+ cells from newborn Sprague-Dawley rats were grown for five days on uncoated dishes in defined medium with or without supplementation of basic fibroblast growth factor (bFGF) and/or T3. Then, PSA-NCAM+ spheres were prepared in single cells and transferred to polyornithine/fibronectin-coated glass coverslips for five days to determine the fate of the cells according to the supplementation of these molecules. T3 responsiveness of ABCD2 was analyzed using real-time quantitative polymerase chain reaction, the growth and fate of cells were determined using 5-bromo-2-deoxyuridine incorporation and immunocytochemistry, respectively.
RESULTS
Results demonstrated that T3 induces overexpression of the ABCD2 gene in PSA-NCAM+ cells, and can enhance PSA-NCAM+ cell growth in the presence of bFGF, favoring an oligodendrocyte fate.
CONCLUSION
These results may provide new insights into investigation of PSA-NCAM+ cells for therapeutic application to X-linked adrenoleukodystrophy.
Keyword
MeSH Terms
-
ATP-Binding Cassette Transporters/*metabolism
Adrenoleukodystrophy/genetics/*therapy
Animals
Animals, Newborn
Bromodeoxyuridine
Cell Differentiation
Fibroblast Growth Factor 2/pharmacology
Fibronectins/metabolism
Immunohistochemistry
Neural Cell Adhesion Molecules/*genetics
Rats
Rats, Sprague-Dawley
Real-Time Polymerase Chain Reaction
Sialic Acids/metabolism
Stem Cells
Thyroid Hormones/*metabolism
Triiodothyronine/pharmacology
Fibronectins
Neural Cell Adhesion Molecules
Sialic Acids
Thyroid Hormones
Triiodothyronine
Fibroblast Growth Factor 2
Bromodeoxyuridine
Figure
Cited by 1 articles
-
Clinical and Genetic Aspects in Twelve Korean Patients with Adrenomyeloneuropathy
Hyung Jun Park, Ha Young Shin, Hoon-Chul Kang, Byung-Ok Choi, Bum Chun Suh, Ho Jin Kim, Young-Chul Choi, Phil Hyu Lee, Seung Min Kim
Yonsei Med J. 2014;55(3):676-682. doi: 10.3349/ymj.2014.55.3.676.
Reference
-
1. Moser HW. Adrenoleukodystrophy: phenotype, genetics, pathogenesis and therapy. Brain. 1997. 120(Pt 8):1485–1508.
Article2. Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009. 326:818–823.
Article3. Duncan ID. Oligodendrocytes and stem cell transplantation: their potential in the treatment of leukoencephalopathies. J Inherit Metab Dis. 2005. 28:357–368.
Article4. Decker L, Avellana-Adalid V, Nait-Oumesmar B, Durbec P, Baron-Van Evercooren A. Oligodendrocyte precursor migration and differentiation: combined effects of PSA residues, growth factors, and substrates. Mol Cell Neurosci. 2000. 16:422–439.
Article5. Grinspan J. Cells and signaling in oligodendrocyte development. J Neuropathol Exp Neurol. 2002. 61:297–306.
Article6. Ben-Hur T, Rogister B, Murray K, Rougon G, Dubois-Dalcq M. Growth and fate of PSA-NCAM+ precursors of the postnatal brain. J Neurosci. 1998. 18:5777–5788.
Article7. Kemp S, Wanders RJ. X-linked adrenoleukodystrophy: very long-chain fatty acid metabolism, ABC half-transporters and the complicated route to treatment. Mol Genet Metab. 2007. 90:268–276.
Article8. Fourcade S, Savary S, Gondcaille C, Berger J, Netik A, Cadepond F, et al. Thyroid hormone induction of the adrenoleukodystrophy-related gene (ABCD2). Mol Pharmacol. 2003. 63:1296–1303.
Article9. Lubetzki C, Goujet-Zalc C, Gansmüller A, Monge M, Brillat A, Zalc B. Morphological, biochemical, and functional characterization of bulk isolated glial progenitor cells. J Neurochem. 1991. 56:671–680.
Article10. Griffin DE, Moser HW, Mendoza Q, Moench TR, O'Toole S, Moser AB. Identification of the inflammatory cells in the central nervous system of patients with adrenoleukodystrophy. Ann Neurol. 1985. 18:660–664.
Article11. Moser HW, Tutschka PJ, Brown FR 3rd, Moser AE, Yeager AM, Singh I, et al. Bone marrow transplant in adrenoleukodystrophy. Neurology. 1984. 34:1410–1417.
Article12. Aubourg P, Blanche S, Jambaqué I, Rocchiccioli F, Kalifa G, Naud-Saudreau C, et al. Reversal of early neurologic and neuroradiologic manifestations of X-linked adrenoleukodystrophy by bone marrow transplantation. N Engl J Med. 1990. 322:1860–1866.
Article13. Korenke GC, Hunneman DH, Kohler J, Stöckler S, Landmark K, Hanefeld F. Glyceroltrioleate/glyceroltrierucate therapy in 16 patients with X-chromosomal adrenoleukodystrophy/adrenomyeloneuropathy: effect on clinical, biochemical and neurophysiological parameters. Eur J Pediatr. 1995. 154:64–70.
Article14. Baas D, Bourbeau D, Carre JL, Sarlieve LL, Dussault JH, Puymirat J. Expression of alpha and beta thyroid receptors during oligodendrocyte differentiation. Neuroreport. 1994. 5:1805–1808.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Allicin Reduces Adhesion Molecules and NO Production Induced by gamma irradiation in Human Endothelial Cells
- The expression of NCAM ( Neural Cell Adhesion Molecule) in myometrium and leiomyoma
- Aberrant L1 Cell Adhesion Molecule Expression in Cancer: In View of Proliferation, Migration and Invasion
- Homophilic interaction of the L1 family of cell adhesion molecules
- Expression of Intercellular Adhesion Molecule-1 and E-Selectin in Gastric Cancer and Their Clinical Significance