Yonsei Med J.
2013 Jan;54(1):123-130. 10.3349/ymj.2013.54.1.123.
Correlations of Dynamic Contrast-Enhanced Magnetic Resonance Imaging with Morphologic, Angiogenic, and Molecular Prognostic Factors in Rectal Cancer
- Affiliations
-
- 1Department of Medicine, Graduate School, Yonsei University, Seoul, Korea.
- 2Department of Pathology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Radiology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. kwkimyd@yuhs.ac
- 4Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Surgery, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 6Department of Radiology, Yeouido St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 7Department of Radiology, Seoul Veterans Hospital, Seoul, Korea.
- KMID: 1776927
- DOI: http://doi.org/10.3349/ymj.2013.54.1.123
Abstract
- PURPOSE
To investigate the correlations between parameters of dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and prognostic factors in rectal cancer.
MATERIALS AND METHODS
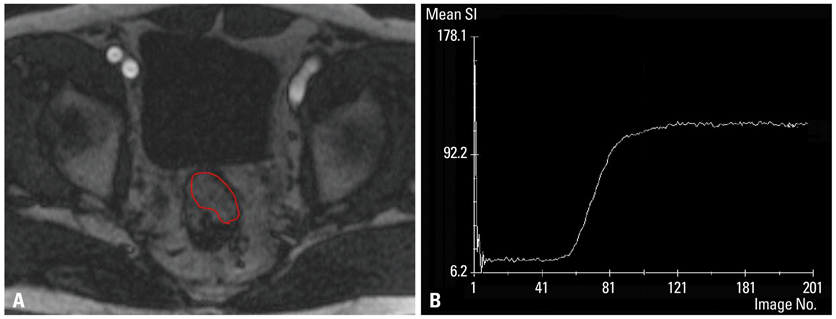
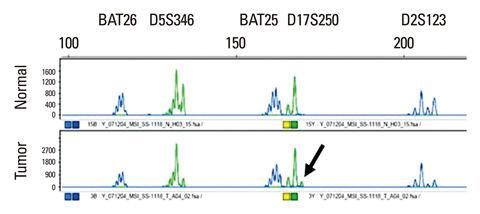
We studied 29 patients with rectal cancer who underwent gadolinium contrast-enhanced, T1-weighted DCE-MRI with a three Tesla scanner prior to surgery. Signal intensity on DCE-MRI was independently measured by two observers to examine reproducibility. A time-signal intensity curve was generated, from which four semiquantitative parameters were calculated: steepest slope (SLP), time to peak (Tp), relative enhancement during a rapid rise (Erise), and maximal enhancement (Emax). Morphologic prognostic factors including T stage, N stage, and histologic grade were identified. Tumor angiogenesis was evaluated in terms of microvessel count (MVC) and microvessel area (MVA) by morphometric study. As molecular factors, the mutation status of the K-ras oncogene and microsatellite instability were assessed. DCE-MRI parameters were correlated with each prognostic factor using bivariate correlation analysis. A p-value of <0.05 was considered significant.
RESULTS
Erise was significantly correlated with N stage (r=-0.387 and -0.393, respectively, for two independent data), and Tp was significantly correlated with histologic grade (r=0.466 and 0.489, respectively). MVA was significantly correlated with SLP (r=-0.532 and -0.535, respectively) and Erise (r=-0.511 and -0.446, respectively). MVC was significantly correlated with Emax (r=-0.435 and -0.386, respectively). No significant correlations were found between DCE-MRI parameters and T stage, K-ras mutation, or microsatellite instability.
CONCLUSION
DCE-MRI may provide useful prognostic information in terms of histologic differentiation and angiogenesis in rectal cancer.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Cell Differentiation
Contrast Media/*pharmacology
DNA Mutational Analysis
Female
Gadolinium/pharmacology
Genes, ras
Humans
Magnetic Resonance Imaging/*methods
Male
Microcirculation
Microsatellite Instability
Middle Aged
Neoplasm Staging
Neovascularization, Pathologic
Prognosis
Rectal Neoplasms/*diagnosis/genetics/*pathology
Retrospective Studies
Time Factors
Contrast Media
Gadolinium
Figure
Reference
-
1. Zlobec I, Lugli A. Prognostic and predictive factors in colorectal cancer. J Clin Pathol. 2008. 61:561–569.
Article2. Takahashi Y, Tucker SL, Kitadai Y, Koura AN, Bucana CD, Cleary KR, et al. Vessel counts and expression of vascular endothelial growth factor as prognostic factors in node-negative colon cancer. Arch Surg. 1997. 132:541–546.
Article3. Takebayashi Y, Aklyama S, Yamada K, Akiba S, Aikou T. Angiogenesis as an unfavorable prognostic factor in human colorectal carcinoma. Cancer. 1996. 78:226–231.
Article4. Saclarides TJ, Speziale NJ, Drab E, Szeluga DJ, Rubin DB. Tumor angiogenesis and rectal carcinoma. Dis Colon Rectum. 1994. 37:921–926.
Article5. Choi HJ, Hyun MS, Jung GJ, Kim SS, Hong SH. Tumor angiogenesis as a prognostic predictor in colorectal carcinoma with special reference to mode of metastasis and recurrence. Oncology. 1998. 55:575–581.
Article6. Benhattar J, Losi L, Chaubert P, Givel JC, Costa J. Prognostic significance of K-ras mutations in colorectal carcinoma. Gastroenterology. 1993. 104:1044–1048.
Article7. Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005. 23:609–618.
Article8. Hylton N. Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J Clin Oncol. 2006. 24:3293–3298.
Article9. George ML, Dzik-Jurasz AS, Padhani AR, Brown G, Tait DM, Eccles SA, et al. Non-invasive methods of assessing angiogenesis and their value in predicting response to treatment in colorectal cancer. Br J Surg. 2001. 88:1628–1636.
Article10. Tuncbilek N, Karakas HM, Altaner S. Dynamic MRI in indirect estimation of microvessel density, histologic grade, and prognosis in colorectal adenocarcinomas. Abdom Imaging. 2004. 29:166–172.
Article11. Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, et al. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer. 2006. 94:1823–1832.
Article12. Finkelstein SD, Sayegh R, Christensen S, Swalsky PA. Genotypic classification of colorectal adenocarcinoma. Biologic behavior correlates with K-ras-2 mutation type. Cancer. 1993. 71:3827–3838.
Article13. Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, et al. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer. 2002. 38:1564–1579.
Article14. Buadu LD, Murakami J, Murayama S, Hashiguchi N, Sakai S, Masuda K, et al. Breast lesions: correlation of contrast medium enhancement patterns on MR images with histopathologic findings and tumor angiogenesis. Radiology. 1996. 200:639–649.
Article15. Hawighorst H, Knapstein PG, Knopp MV, Vaupel P, van Kaick G. Cervical carcinoma: standard and pharmacokinetic analysis of time-intensity curves for assessment of tumor angiogenesis and patient survival. MAGMA. 1999. 8:55–62.
Article16. Leach MO. Application of magnetic resonance imaging to angiogenesis in breast cancer. Breast Cancer Res. 2001. 3:22–27.17. Miller JC, Pien HH, Sahani D, Sorensen AG, Thrall JH. Imaging angiogenesis: applications and potential for drug development. J Natl Cancer Inst. 2005. 97:172–187.
Article18. Rehman S, Jayson GC. Molecular imaging of antiangiogenic agents. Oncologist. 2005. 10:92–103.
Article19. Park HC, Shimizu S, Yonesaka A, Tsuchiya K, Ebina Y, Taguchi H, et al. High dose three-dimensional conformal boost using the real-time tumor tracking radiotherapy system in cervical cancer patients unable to receive intracavitary brachytherapy. Yonsei Med J. 2010. 51:93–99.
Article20. Hawighorst H, Knapstein PG, Weikel W, Knopp MV, Zuna I, Knof A, et al. Angiogenesis of uterine cervical carcinoma: characterization by pharmacokinetic magnetic resonance parameters and histological microvessel density with correlation to lymphatic involvement. Cancer Res. 1997. 57:4777–4786.21. Schlemmer HP, Merkle J, Grobholz R, Jaeger T, Michel MS, Werner A, et al. Can pre-operative contrast-enhanced dynamic MR imaging for prostate cancer predict microvessel density in prostatectomy specimens? Eur Radiol. 2004. 14:309–317.
Article22. Goh V, Padhani AR, Rasheed S. Functional imaging of colorectal cancer angiogenesis. Lancet Oncol. 2007. 8:245–255.
Article23. Galbraith SM, Lodge MA, Taylor NJ, Rustin GJ, Bentzen S, Stirling JJ, et al. Reproducibility of dynamic contrast-enhanced MRI in human muscle and tumours: comparison of quantitative and semi-quantitative analysis. NMR Biomed. 2002. 15:132–142.
Article24. Gu J, Khong PL, Wang S, Chan Q, Wu EX, Law W, et al. Dynamic contrast-enhanced MRI of primary rectal cancer: quantitative correlation with positron emission tomography/computed tomography. J Magn Reson Imaging. 2011. 33:340–347.
Article25. Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999. 10:223–232.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dynamic Contrast-Enhanced MR Imaging of Tietze’s Syndrome: a Case Report
- Perfusion Parameters of Dynamic Contrast-Enhanced Magnetic Resonance Imaging in Patients with Rectal Cancer: Correlation with Microvascular Density and Vascular Endothelial Growth Factor Expression
- Imaging Diagnosis of Colorectal Cancer
- Multidisciplinary Functional MR Imaging for Prostate Cancer
- Prostate Cancer: Added Value of Subtraction Dynamic Imaging in 3T Magnetic Resonance Imaging with a Phased-array Body Coil