Yonsei Med J.
2008 Oct;49(5):765-774. 10.3349/ymj.2008.49.5.765.
Prostate Cancer: Added Value of Subtraction Dynamic Imaging in 3T Magnetic Resonance Imaging with a Phased-array Body Coil
- Affiliations
-
- 1Department of Radiology, Yonsei University College of Medicine, Yongdong Severance Hospital, Seoul, Korea. yjsrad97@yuhs.ac
- 2Department of Pathology, Yonsei University College of Medicine, Yongdong Severance Hospital, Seoul, Korea.
- 3Department of Urology, Yonsei University College of Medicine, Yongdong Severance Hospital, Seoul, Korea.
- KMID: 2158195
- DOI: http://doi.org/10.3349/ymj.2008.49.5.765
Abstract
- PURPOSE
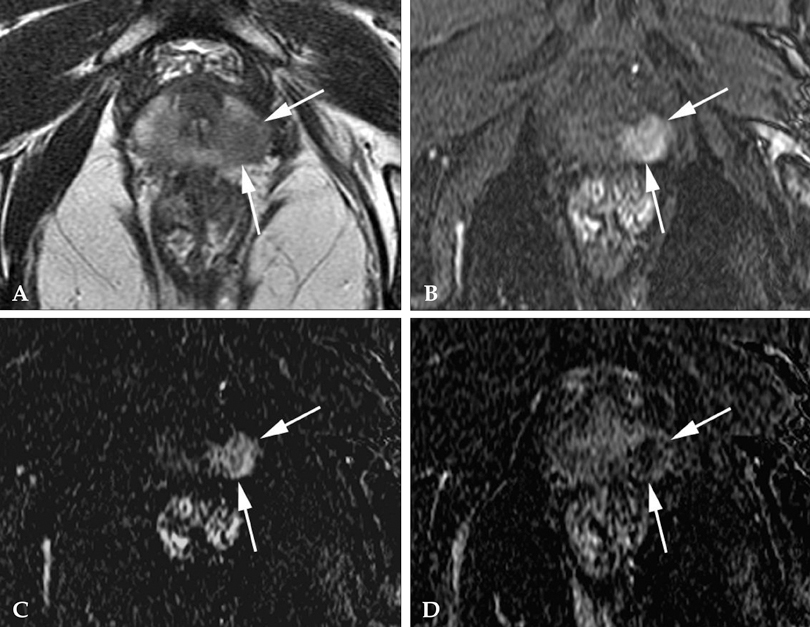
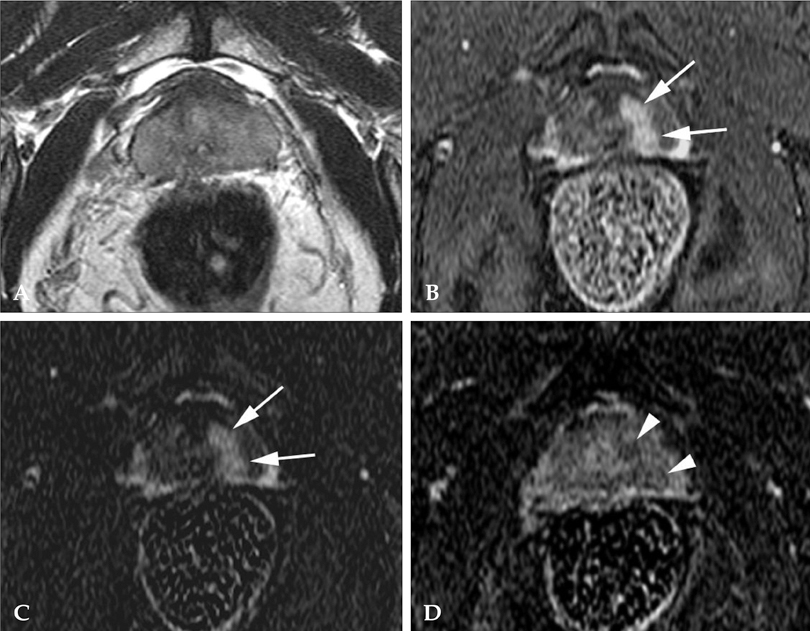
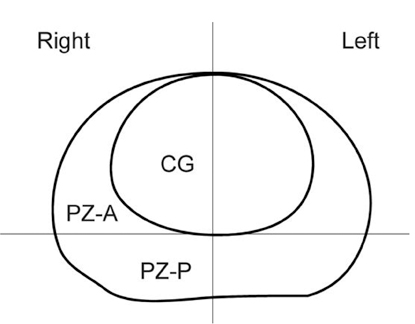
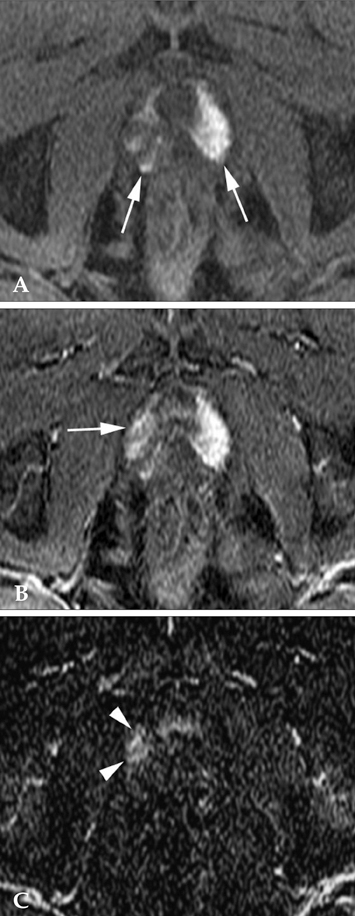
To determine the added value of dynamic subtraction magnetic resonance (MR) imaging for the localization of prostate cancer. MATERIALS AND METHODS: We examined 21 consecutive patients who underwent MR imaging in 3T unit with a phased-array body coil and then had radical prostatectomy. After T2-weighted fast spin-echo imaging, we performed a contrast-enhanced dynamic 3D gradient-echo imaging consisting of pre-contrast, 2 successive early-phased (first imaging was started just after the appearance of contrast material in the aortic bifurcation followed by second imaging 35 seconds after the initiation of first imaging) and one 5-minute delayed post-contrast series. Subtraction of pre-contrast images from corresponding post-contrast images of each phase was performed on the console. RESULTS: On ROC analysis, the overall accuracy (Az value) of dynamic imaging combined with subtraction imaging was higher than T2-weighted imaging (p = 0.001) or conventional dynamic imaging alone (p = 0.074) for localization of cancer foci regardless of their zonal locations. Among pathologically verified 81 lesions, the mean volume of detected lesions with the subtraction images (n = 49, 0.69cm3) was smaller than with T2-weighted images (n = 14, 1.05cm3) or conventional dynamic images (n = 43, 0.71cm(3)). CONCLUSION: For localization of small prostate cancer, additional subtraction for the dynamic imaging could be superior to both T2-weighted imaging and un-subtracted dynamic imaging.
MeSH Terms
Figure
Reference
-
1. Schiebler ML, Schnall MD, Pollack HM, Lenkinski RE, Tomaszewski JE, Wein AJ, et al. Current role of MR imaging in the staging of adenocarcinoma of the prostate. Radiology. 1993. 189:339–352.
Article2. Jager GJ, Severens JL, Thornbury JR, de La Rosette JJ, Ruijs SH, Barentsz JO. Prostate cancer staging: should MR imaging be used? A decision analytic approach. Radiology. 2000. 215:445–451.
Article3. Sosna J, Pedrosa I, Dewolf WC, Mahallati H, Lenkinski RE, Rofsky NM. MR imaging of the prostate at 3 Tesla: comparison of an external phased-array coil to imaging with an endorectal coil at 1.5 Tesla. Acad Radiol. 2004. 11:857–862.4. Quint LE, Van Erp JS, Bland PH, Del Buono EA, Mandell SH, Grossman HB, et al. Prostate cancer: correlation of MR images with tissue optical density at pathologic examination. Radiology. 1991. 179:837–842.
Article5. Engelbrecht MR, Huisman HJ, Laheij RJ, Jager GJ, van Leenders GJ, Hulsbergen-Van De Kaa CA, et al. Discrimination of prostate cancer from normal peripheral zone and central gland tissue by using dynamic contrast-enhanced MR imaging. Radiology. 2003. 229:248–254.
Article6. White S, Hricak H, Forstner R, Kurhanewicz J, Vigneron DB, Zaloudek CJ, et al. Prostate cancer: effect of postbiopsy hemorrhage on interpretation of MR images. Radiology. 1995. 195:385–390.
Article7. Brown G, Macvicar DA, Ayton V, Husband JE. The role of intravenous contrast enhancement in magnetic resonance imaging of prostatic carcinoma. Clin Radiol. 1995. 50:601–606.
Article8. Kim CK, Park BK, Kim B. Localization of prostate cancer using 3T MRI: comparison of T2-weighted and dynamic contrast-enhanced imaging. J Comput Assist Tomogr. 2006. 30:7–11.9. Jager GJ, Ruijter ET, van de Kaa CA, de la Rosette JJ, Oosterhof GO, Thornbury JR, et al. Dynamic TurboFLASH subtraction technique for contrast-enhanced MR imaging of the prostate: correlation with histopathologic results. Radiology. 1997. 203:645–652.
Article10. Lovett K, Rifkin MD, McCue PA, Choi H. MR imaging characteristics of noncancerous lesions of the prostate. J Magn Reson Imaging. 1992. 2:35–39.
Article11. Carter HB, Brem RF, Tempany CM, Yang A, Epstein JI, Walsh PC, et al. Nonpalpable prostate cancer: detection with MR imaging. Radiology. 1991. 178:523–525.
Article12. Li H, Sugimura K, Kaji Y, Kitamura Y, Fujii M, Hara I, et al. Conventional MRI capabilities in the diagnosis of prostate cancer in the transition zone. AJR Am J Roentgenol. 2006. 186:729–742.
Article13. Stamey TA, Freiha FS, McNeal JE, Redwine EA, Whittemore AS, Schmid HP. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer. 1993. 71:3 Suppl. 933–938.
Article14. Ogura K, Maekawa S, Okubo K, Aoki Y, Okada T, Oda K, et al. Dynamic endorectal magnetic resonance imaging for local staging and detection of neurovascular bundle involvement of prostate cancer: correlation with histopathologic results. Urology. 2001. 57:721–726.
Article15. Fütterer JJ, Engelbrecht MR, Huisman HJ, Jager GJ, Hulsbergen-van De Kaa CA, Witjes JA, et al. Staging prostate cancer with dynamic contrast-enhanced endorectal MR imaging prior to radical prostatectomy: experienced versus less experienced readers. Radiology. 2005. 237:541–549.16. Gilles R, Guinebretière JM, Shapeero LG, Lesnik A, Contesso G, Sarrazin D, et al. Assessment of breast cancer recurrence with contrast-enhanced subtraction MR imaging: preliminary results in 26 patients. Radiology. 1993. 188:473–478.17. Yu JS, Rofsky NM. Dynamic subtraction MR imaging of the liver: advantages and pitfalls. AJR Am J Roentgenol. 2003. 180:1351–1357.
Article18. Turnbull LW, Buckley DL, Turnbull LS, Liney GP, Knowles AJ. Differentiation of prostatic carcinoma and benign prostatic hyperplasia: correlation between dynamic Gd-DTPA-enhanced MR imaging and histopathology. J Magn Reson Imaging. 1999. 9:311–316.
Article19. Shukla-Dave A, Hricak H, Eberhardt SC, Olgac S, Muruganandham M, Scardino PT, et al. Chronic prostatitis: MR imaging and 1H MR spectroscopic imaging findings-initial observations. Radiology. 2004. 231:717–724.
Article20. Kim CK, Park BK, Lee HM, Kwon GY. Value of diffusion-weighted imaging for the prediction of prostate cancer location at 3T using a phased-array coil: preliminary results. Invest Radiol. 2007. 42:842–847.
Article21. Girouin N, Mège-Lechevallier F, Tonina Senes A, Bissery A, Rabilloud M, Maréchal JM, et al. Prostate dynamic contrast-enhanced MRI with simple visual diagnostic criteria: is it reasonable? Eur Radiol. 2007. 17:1498–1509.
Article22. Namimoto T, Morishita S, Saitoh R, Kudoh J, Yamashita Y, Takahashi M. The value of dynamic MR imaging for hypointensity lesions of the peripheral zone of the prostate. Comput Med Imaging Graph. 1998. 22:239–245.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Pelvic Phased-Array versus Endorectal Coil Magnetic Resonance Imaging at 3 Tesla for Local Staging of Prostate Cancer
- Reliability of MRI Using Endorectal Coil in Local Staging of Prostate Carcinoma
- A Tool Box to Evaluate the Phased Array Coil Performance Using Retrospective 3D Coil Modeling
- MR Evaluation of Rectal Carcinoma: Pelvic Phased-Array Coil versus Endorectal-Pelvic Phased-Array Coil
- A Projection-based Intensity Correction Method of Phased-Array Coil Images