Korean J Radiol.
2008 Jun;9(3):196-204. 10.3348/kjr.2008.9.3.196.
Tracking of Neural Stem Cells in Rats with Intracerebral Hemorrhage by the Use of 3T MRI
- Affiliations
-
- 1Department of Radiology, Chonnam National University Medical School, Gwang-ju, Korea. yjeong@jnu.ac.kr
- 2Department of Physiology, Chonnam National University Medical School, Gwang-ju, Korea.
- 3Department of Pathology, Chonnam National University Medical School, Gwang-ju, Korea.
- KMID: 1758452
- DOI: http://doi.org/10.3348/kjr.2008.9.3.196
Abstract
OBJECTIVE
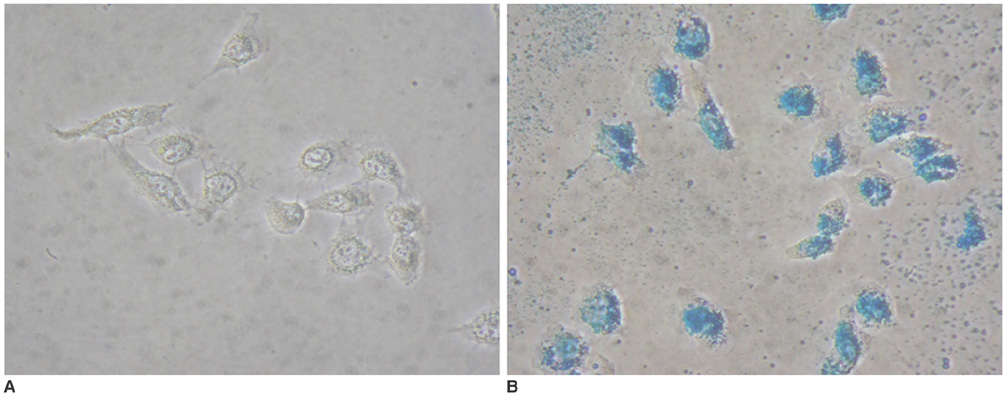
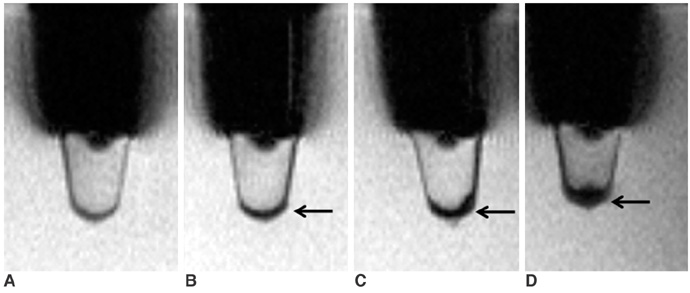
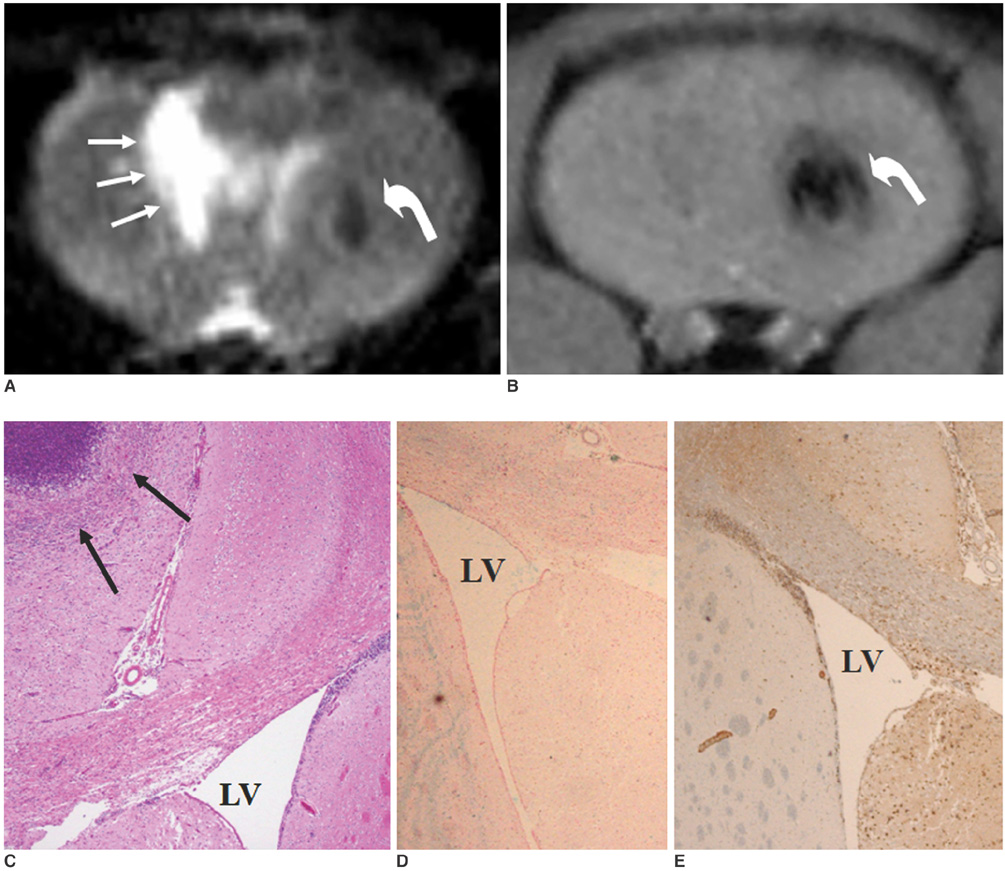
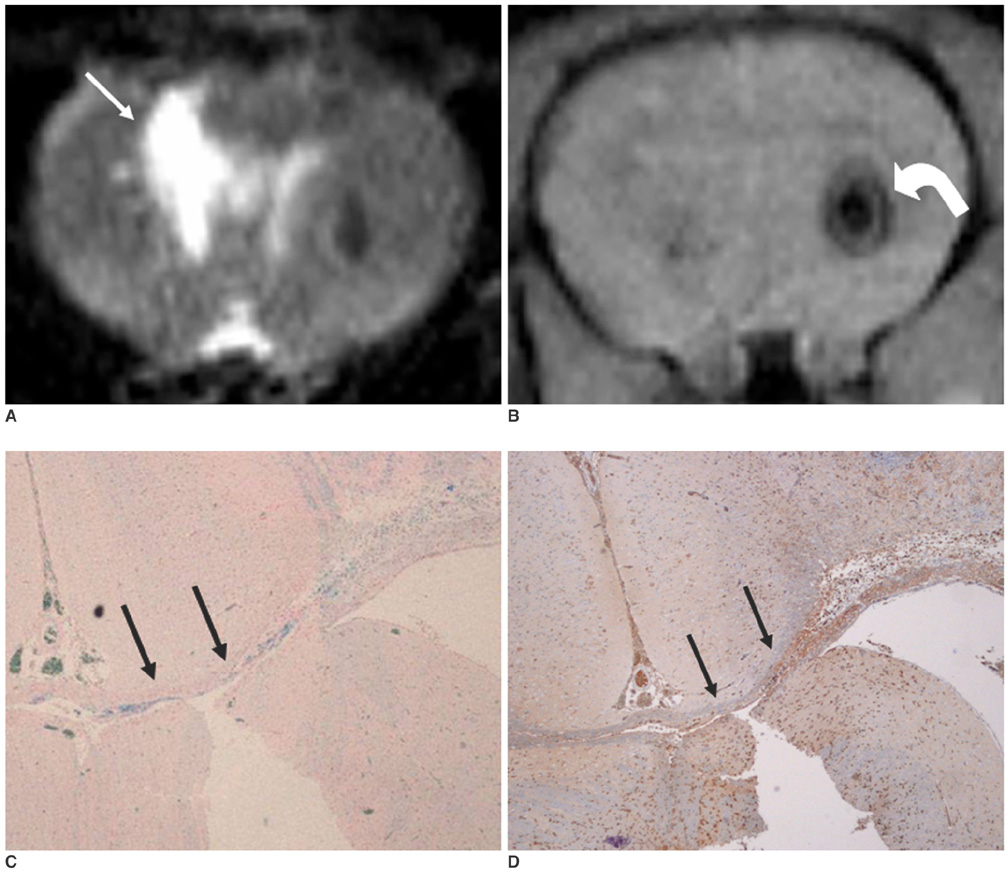
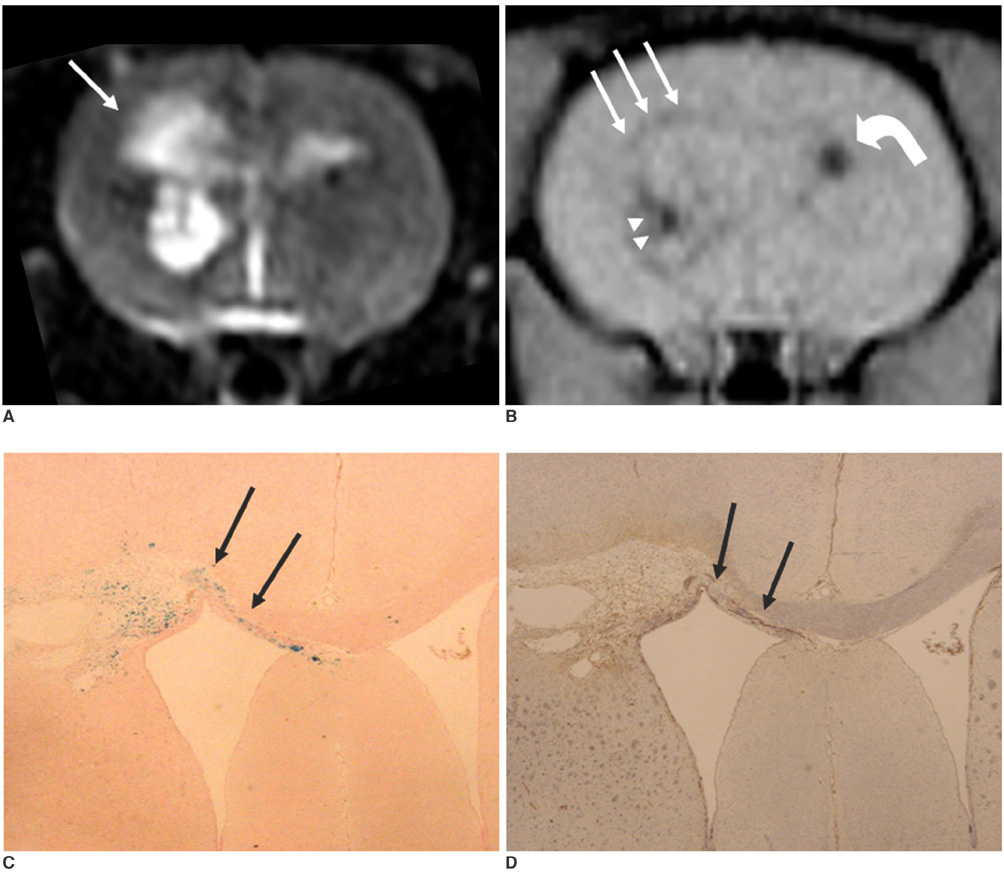
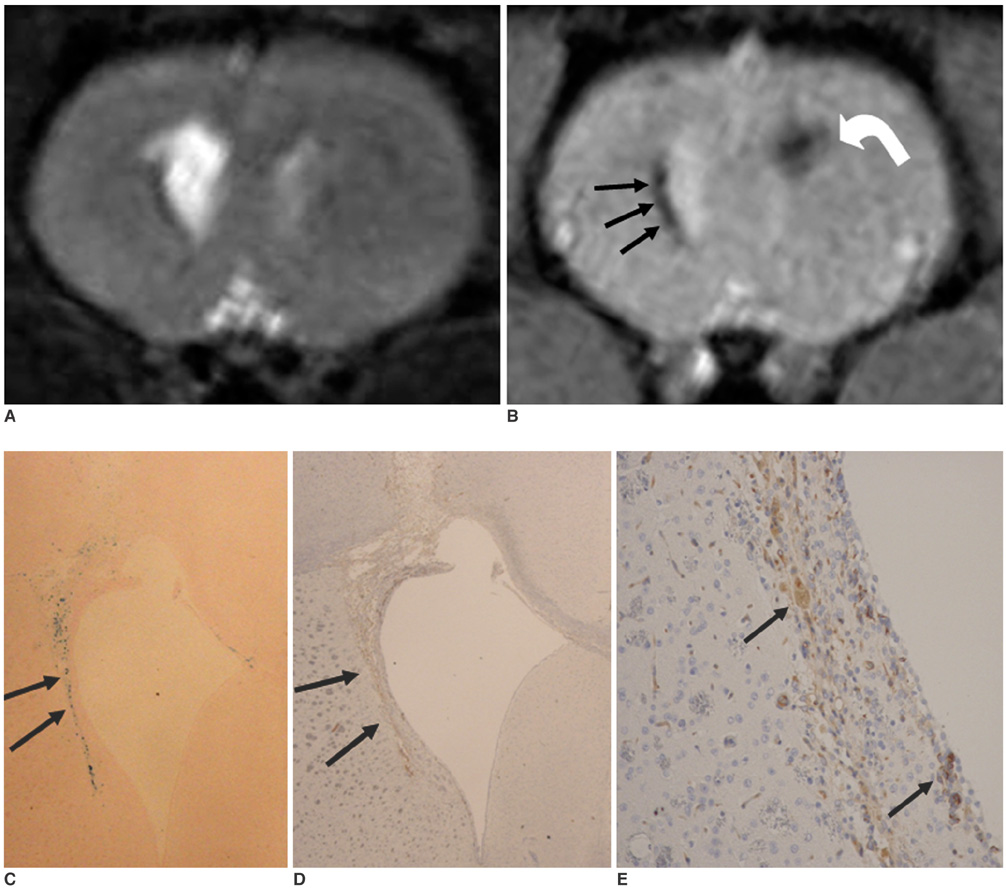
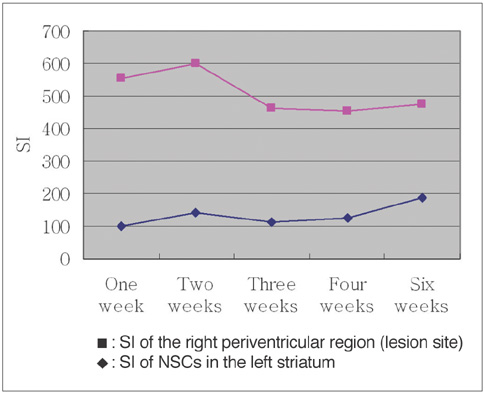
To access the feasibility of clinically available 3T MRI to detect the migration of labeled neural stem cells (NSCs) in intracerebral hemorrhage (ICH) in a rat model. MATERIALS AND METHODS: The ethics committee of our institution approved this study. ICH was induced by the injection of collagenase type IV into the right striatum of ten Sprague-Dawley rats. Human NSCs conjugated with Feridex (super-paramagnetic iron oxide: SPIO) were transplanted into the left striatum one week after ICH induction. MRI was performed on a 3T scanner during the first, second, third, fourth, and sixth weeks post-transplantation. MRI was obtained using coronal T2- and T2*-weighted sequences. Two rats were sacrificed every week after in vivo MRI in order to analyze the histological findings. RESULTS: ICH in the right striatum was detected by MRI one and two weeks after transplantation without migration of the NSCs. There was no migration of the NSCs as seen on the histological findings one week after transplantation. The histological findings two weeks after transplantation showed a small number of NSCs along the corpus callosum. On MRI three weeks after transplantation, there was a hypointense line along the corpus callosum and decreased signal intensity in the right periventricular region. Histological findings three weeks after transplantation confirmed the presence of the hypointense line representing SPIO-labeled NSCs. MRI four and six weeks after transplantation showed a hypointense spot in the right periventricular region. The histological findings four and six weeks after transplantation showed the presence of prominent NSCs in the right periventricular region. CONCLUSION: 3T MRI can detect the migration of NSCs in rats with ICH along the corpus callosum. Therefore, 3T MRI could be feasible for detecting the migration of NSCs in the clinical setting of stem cell therapy.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
A Study of Feasibility of Brain Imaging in Medium- and Small-Sized Animals: Using a Clinical 3T MR System with Three Surface Coils
Shin Young Park, Mi Ri Jeong, Byung Mann Cho, Kang Soo Kim, Hak Jin Kim
J Korean Soc Radiol. 2017;77(5):317-326. doi: 10.3348/jksr.2017.77.5.317.
Reference
-
1. Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000. 97:12846–12851.2. Modo M, Stroemer RP, Tang E, Patel S, Hodges H. Effects of implantation site of stem cell grafts on behavioral recovery from stroke damage. Stroke. 2002. 33:2270–2278.3. Bjorklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nat Neurosci. 2000. 3:537–544.4. Savitz SI, Rosenbaum DM, Dinsmore JH, Wechsler LR, Caplan LR. Cell transplantation for stroke. Ann Neurol. 2002. 52:266–275.5. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001. 344:1450–1460.6. Lo YK, Yiu Ch, Hu HH, Su MS, Laeuchli SC. Frequency and characteristics of early seizures in Chinese acute stroke. Acta Neurol Scand. 1994. 90:83–85.7. Jeong SW, Chu K, Jung KH, Kim SU, Kim M, Roh JK. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke. 2003. 34:2258–2263.8. Kim DE, Schellingerhout D, Ishii K, Shah K, Weissleder R. Imaging of stem cell recruitment to ischemic infarcts in a murine model. Stroke. 2004. 35:952–957.9. Hoehn M, Kustermann E, Blunk J, Wiedermann D, Trapp T, Wecker S, et al. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental sroke in rat. Proc Natl Acad Sci USA. 2002. 99:16267–16272.10. Magnitsky S, Watson DJ, Walton RM, Pickup S, Bulte JW, Wolfe JH, et al. In vivo and ex vivo MRI detection of localized and disseminated neural stem cell grafts in the mouse brain. Neuroimage. 2005. 26:744–754.11. Modo M, Mellodew K, Cash D, Fraser SE, Meade TJ, Price J, et al. Mapping transplanted stem cell migration after a stroke: a serial, in vivo magnetic resonance imaging study. Neuroimage. 2004. 21:311–317.12. Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005. 57:874–882.13. Zhang Z, Ziang Q, Jaing F, Ding G, Zhang R, Wang L, et al. In vivo magnetic resonance imaging tracks adult neural progenitor cell targeting of brain tumor. Neuroimage. 2004. 23:281–287.14. Frank JA, Miller BR, Arbab AS, Zuwicke H, Jordan EK, Lewis BK, et al. Clinically applicable labeling of mammalian and stem cells by combining superparamagnetic iron oxides and transfection agents. Radiology. 2003. 228:480–487.15. Bulte JW, Duncan ID, Frank JA. In vivo magnetic resonance tracking of magnetically labeled cells after transplantation. J Cereb Blood Flow Metab. 2002. 22:899–907.16. Stroh A, Faber C, Neuberger T, Lorenz P, Sieland K, Jakob PM, et al. In vivo detection limits of magnetically labeled embryonic stem cells in the rat brain using high-field (17.6 T) magnetic resonance imaging. Neuroimage. 2005. 24:635–645.17. Zhang ZG, Jiang Q, Zhang R, Zhang L, Wang L, Zhang L, et al. Magnetic resonance imaging and neurosphere therapy of stroke in rat. Ann Neurol. 2003. 53:259–263.18. Bulte JW, Zhang S, van Gelderen P, Herynek V, Jordan EK, Duncan ID, et al. Neurotransplantation of magnetically labeled oligodendrocyte progenitors: magnetic resonance tracking of cell migration and myelination. Proc Natl Acad Sci USA. 1999. 96:15256–15261.19. Sipe JC, Filippi M, Martino G, Furlan R, Rocca MA, Rovaris M, et al. Method for intracellular magnetic labeling of human mononuclear cells using approved iron contrast agents. Magn Reson Imaging. 1999. 17:1521–1523.20. de Laquintane BD, Dousset V, Solanilla A, Petry KG, Ripoche J. Iron particle labeling of haematopoietic progenitor cells: an in vitro study. Biosci Rep. 2002. 22:549–554.21. Arnold LJ Jr, Dagan A, Gutheil J, Kaplan NO. Antineoplastic activity of poly(L-Lysin) with some ascites tumor cells. Proc Natl Acad Sci USA. 1979. 76:3246–3250.22. Sorgi FL, Bhattacharya S, Huang L. Protamine sulfate enhances lipid-mediated gene transfer. Gene Ther. 1997. 4:961–968.23. Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA. 2004. 101:11839–11844.24. Muller FJ, Snyder EY, Loring JF. Gene therapy: can neural stem cells deliver? Nat Rev Neurosci. 2006. 7:75–84.25. Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001. 32:1005–1011.26. Eglitis MA, Dawson D, Park KW, Mouradian MM. Targeting of marrow-derived astrocytes to the ischemic brain. Neuroreport. 1999. 10:1289–1292.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tracking of Stem Cells for Treatment in Cardiovascular Disease
- Human Neural Stem Cells Transplantation in Experimental Intracerebral Hemorrhage
- Endogenous Neural Stem Cell Proliferation after Intracerebral Hemorrhage in Rat Model
- Combination Cell Therapy with Mesenchymal Stem Cells and Neural Stem Cells for Brain Stroke in Rats
- In vivo Tracking of Human Neural Stem Cells Following Transplantation into a Rodent Model of Ischemic Stroke