Cancer Res Treat.
2005 Feb;37(1):71-77.
Identification of CLP36 as a Tumor Antigen that Induces an Antibody Response in Pancreatic Cancer
- Affiliations
-
- 1Department of Dental Microbiology, Kyungpook National University School of Dentistry, Daegu, Korea. hongsu@knu.ac.kr
Abstract
- PURPOSE
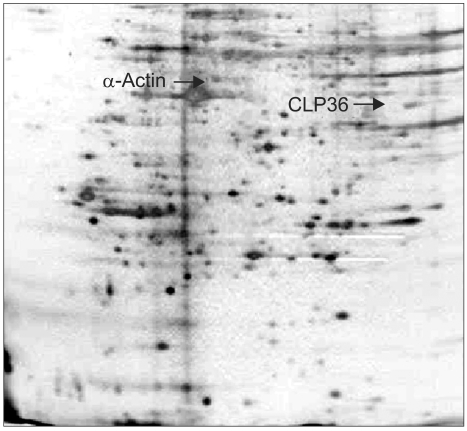
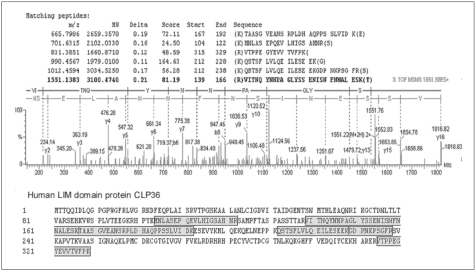
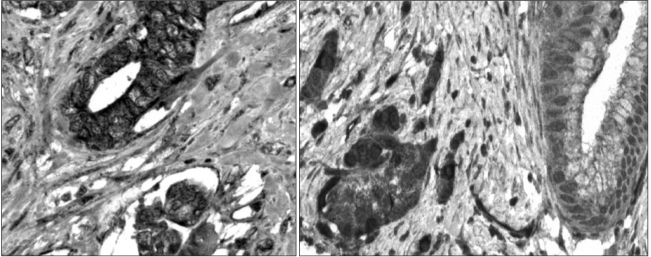
Pancreatic cancer has a poor prognosis, due in part to the lack of an effective approach for its early detection. The identification of tumor antigens potentially provides a means for the early diagnosis. The purpose of this study was to use a proteomic approach for the identification of proteins that commonly induce a humoral response in patients with pancreatic cancer. MATERIALS AND METHODS: Proteins from the pancreatic adenocarcinoma cell line, BxPC3, were subjected to two-dimensional polyacrylamide gel electrophoresis, followed by Western blot analysis, where individual sera were tested for autoantibodies. Sera from 36 patients with pancreatic adenocarcinoma, and 68 from control groups (14 from lung adenocarcinoma, 19 from colon adenocarcinoma and 35 from healthy subjects) were analyzed. CLP36 expression was evaluated by immunohistochemical analysis and real-time PCR. The cellular localization of CLP36 as an autoantigen was investigated by Western blot analysis. RESULTS: The autoantibody was detected against a protein, identified by mass spectrometry as CLP36, in 14 of the 36 sera (38.9%) from patients with a pancreatic adenocarcinoma, and 3 of the 68 controls (4.4%). Immunohistochemical analysis of CLP36 in a tissue array demonstrated diffuse and consistent immunoreactivity in the pancreatic adenocarcinomas. The levels of CLP36 mRNA were highest in the pancreatic cancer cell lines of the different cells analyzed. The molecular weight of the protein displayed in the membrane-rich fraction was larger than that in the cytosolic fraction, which is likely attributable to a post-translational modification. CONCLUSION: CLP36 was identified as a tumor autoantigen inducing a humoral immune response in pancreatic adenocarcinomas. More detailed studies need to be undertaken to understand whether the humoral response by CLP36 is tumor-specific.
MeSH Terms
-
Adenocarcinoma
Antibody Formation*
Antigens, Neoplasm
Autoantibodies
Autoantigens
Blotting, Western
Cell Line
Colon
Cytosol
Early Diagnosis
Electrophoresis, Gel, Two-Dimensional
Electrophoresis, Polyacrylamide Gel
Humans
Immunity, Humoral
Lung
Mass Spectrometry
Molecular Weight
Pancreatic Neoplasms*
Prognosis
Protein Processing, Post-Translational
Proteomics
Real-Time Polymerase Chain Reaction
RNA, Messenger
Antigens, Neoplasm
Autoantibodies
Autoantigens
RNA, Messenger
Figure
Reference
-
1. Brichory FM, Misek DE, Yim AM, Krause MC, Giordano TJ, Beer DG, et al. An immune response manifested by the common occurrence of annexins I and II autoantibodies and high circulating levels of IL-6 in lung cancer. Proc Natl Acad Sci USA. 2001; 98:9824–9829. PMID: 11504947.
Article2. Le Naour F, Misek DE, Krause MC, Deneux L, Giordano TJ, Scholl S, et al. Proteomics-based identification of RS/DJ-1 as a novel circulating tumor antigen in breast cancer. Clin Cancer Res. 2001; 7:3328–3335. PMID: 11705844.3. Stockert E, Jager E, Chen YT, Scanlan MJ, Gout I, Karbach J, et al. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med. 1998; 187:1349–1354. PMID: 9547346.
Article4. Hanash S. Harnessing immunity for cancer marker discovery. Nat Biotechnol. 2003; 21:37–38. PMID: 12511908.
Article5. Ben-Mahrez K, Sorokine I, Thierry D, Kawasumi T, Ishii S, Salmon R, et al. Circulating antibodies against c-myc oncogene product in sera of colorectal cancer patients. Int J Cancer. 1990; 46:35–38. PMID: 2142141.
Article6. Pupa SM, Menard S, Andreola S, Colnaghi MI. Antibody response against the c-erbB-2 oncoprotein in breast carcinoma patients. Cancer Res. 1993; 53:5864–5866. PMID: 7903196.7. Winter SF, Minna JD, Johnson BE, Takahashi T, Gazdar AF, Carbone DP. Development of antibodies against p53 in lung cancer patients appears to be dependent on the type of p53 mutation. Cancer Res. 1992; 52:4168–4174. PMID: 1322237.8. Raedle J, Oremek G, Welker M, Roth WK, Caspary WF, Zeuzem S. p53 autoantibodies in patients with pancreatitis and pancreatic carcinoma. Pancreas. 1996; 13:241–246. PMID: 8884844.
Article9. Maacke H, Hundertmark C, Miska S, Voss M, Kalthoff H, Sturzbecher HW. Autoantibodies in sera of pancreatic cancer patients identify recombination factor Rad51 as a tumour-associated antigen. J Cancer Res Clin Oncol. 2002; 128:219–222. PMID: 11935313.
Article10. Hanash SM, Strahler JR. Advances in two-dimensional electrophoresis. Nature. 1989; 337:485–486. PMID: 2915694.
Article11. Gharahdaghi F, Weinberg CR, Meagher DA, Imai BS, Mische SM. Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: a method for the removal of silver ions to enhance sensitivity. Electrophoresis. 1999; 20:601–605. PMID: 10217175.
Article12. Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998; 4:844–847. PMID: 9662379.
Article13. Feuerstein R, Wang X, Song D, Cooke NE, Liebhaber SA. The LIM/double zinc-finger motif functions as a protein dimerization domain. Proc Natl Acad Sci USA. 1994; 91:10655–10659. PMID: 7938009.
Article14. Dawid IB, Breen JJ, Toyama R. LIM domains: multiple roles as adapters and functional modifiers in protein interactions. Trends Genet. 1998; 14:156–162. PMID: 9594664.
Article15. Dawid IB, Toyama R, Taira M. LIM domain proteins. C R Acad Sci III. 1995; 318:295–306. PMID: 7788499.16. Kuroda S, Tokunaga C, Kiyohara Y, Higuchi O, Konishi H, Mizuno K, et al. Protein-protein interaction of zinc finger LIM domains with protein kinase C. J Biol Chem. 1996; 271:31029–31032. PMID: 8940095.
Article17. Vallenius T, Luukko K, Makela TP. CLP-36 PDZ-LIM protein associates with nonmuscle alpha-actinin-1 and alpha-actinin-4. J Biol Chem. 2000; 275:11100–11105. PMID: 10753915.18. Honda K, Yamada T, Endo R, Ino Y, Gotoh M, Tsuda H, et al. Actinin-4, a novel actin-bundling protein associated with cell motility and cancer invasion. J Cell Biol. 1998; 140:1383–1393. PMID: 9508771.
Article19. Echchakir H, Mami-Chouaib F, Vergnon I, Baurain JF, Karanikas V, Chouaib S, et al. A point mutation in the alpha-actinin-4 gene generates an antigenic peptide recognized by autologous cytolytic T lymphocytes on a human lung carcinoma. Cancer Res. 2001; 61:4078–4083. PMID: 11358829.20. Maggiore G, Veber F, Bernard O, Hadchouel M, Homberg JC, Alvarez F, et al. Autoimmune hepatitis associated with anti-actin antibodies in children and adolescents. J Pediatr Gastroenterol Nutr. 1993; 17:376–381. PMID: 8145091.
Article21. Leibovitch L, George J, Levi Y, Bakimer R, Shoenfeld Y. Anti-actin antibodies in sera from patients with autoimmune liver diseases and patients with carcinomas by ELISA. Immunol Lett. 1995; 48:129–132. PMID: 8719111.
Article22. Konstandoulakis MM, Syrigos KN, Leandros M, Charalabopoulos A, Manouras A, Golematis BC. Autoantibodies in the serum of patients with gastric cancer: their prognostic importance. Hybridoma. 1998; 17:431–435. PMID: 9873988.
Article23. Rosen A, Casciola-Rosen L. Autoantigens as substrates for apoptotic proteases: implications for the pathogenesis of systemic autoimmune disease. Cell Death Differ. 1999; 6:6–12. PMID: 10200542.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Assessment of Asymptomatically Increased CA 19-9
- A Case Report of Anti-f(ce) Identified in a Patient with Pancreatic Cancer
- Delayed radiation-induced inflammation accompanying a marked carbohydrate antigen 19-9 elevation in a patient with resected pancreatic cancer
- Anti-tumor effects of Toxoplasma gondii and antigen-pulsed dendritic cells in mice bearing breast cancer
- Antibody-Drug Conjugates in Head and Neck Cancer