J Vet Sci.
2013 Sep;14(3):281-289. 10.4142/jvs.2013.14.3.281.
Gossypol acetic acid induces apoptosis in RAW264.7 cells via a caspase-dependent mitochondrial signaling pathway
- Affiliations
-
- 1College of Veterinary Medicine, Hunan Agricultural University, Changsha 410128, China. dykeyan3618@163.com
- 2Clinical Stem Cell Research Center, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200127, China.
- 3State Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200032, China.
- KMID: 1705558
- DOI: http://doi.org/10.4142/jvs.2013.14.3.281
Abstract
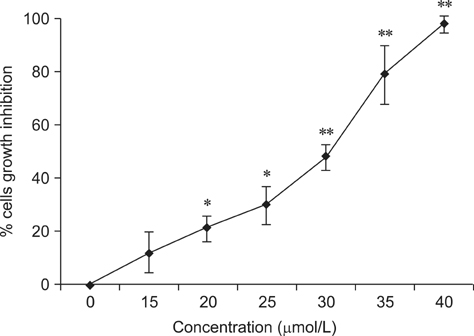
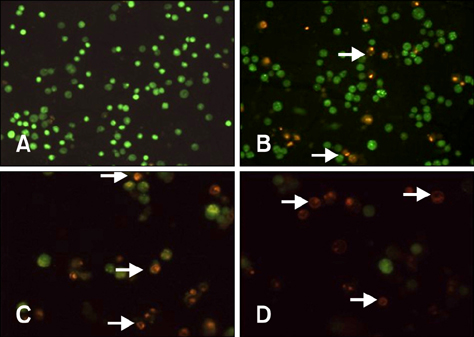
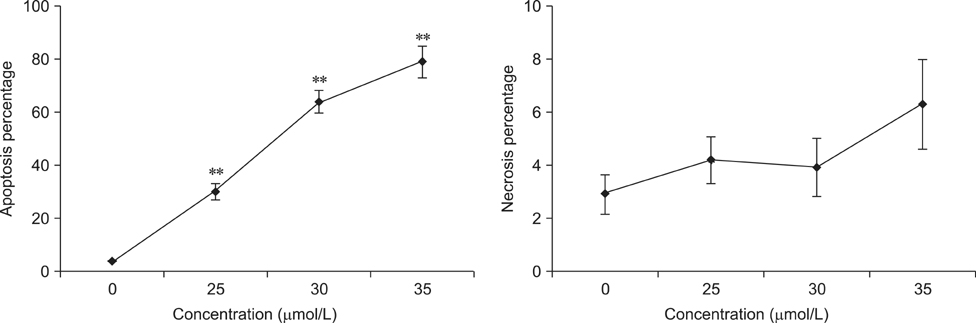
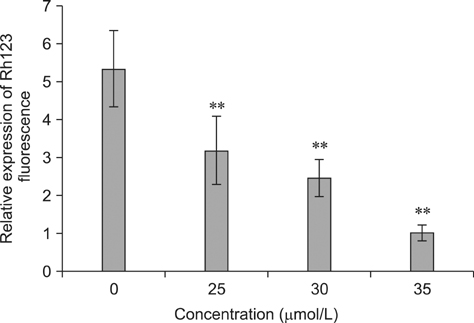
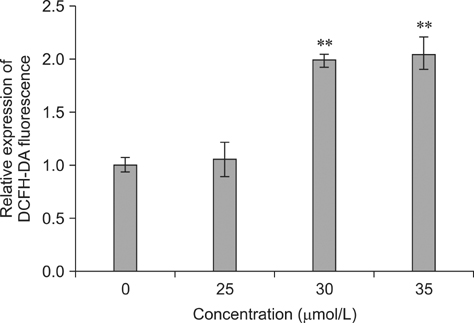
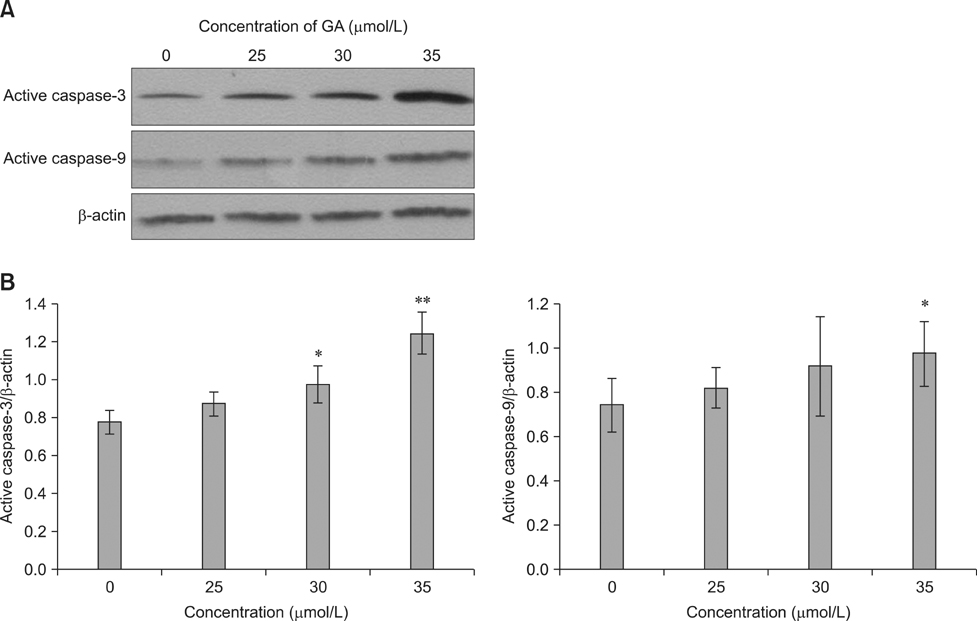
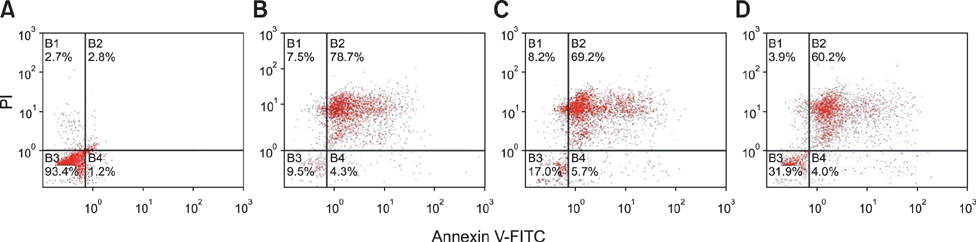
- To investigate the effects of gossypol acetic acid (GA) on proliferation and apoptosis of the macrophage cell line RAW264.7 and further understand the possible underlying mechanism responsible for GA-induced cell apoptosis, RAW264.7 cells were treated with GA (25~35 micromol/L) for 24 h and the cytotoxicity was determined by MTT assay, while apoptotic cells were identified by TUNEL assay, acridine orange/ethidium bromide staining and flow cytometry. Moreover, mitochondrial membrane potential (DeltaPsi(m)) with Rhodamine 123 and reactive oxygen species (ROS) with DCFH-DA were analyzed by fluorescence spectrofluorometry. In addition, the expression of caspase-3 and caspase-9 was assessed by Western Blot assay. Finally, the GA-induced cell apoptosis was evaluated by flow cytometry in the present of caspase inhibitors Z-VAD-FMK and Ac-LEHD-FMK, respectively. GA significantly inhibited the proliferation of RAW264.7 cells in a dose-dependent manner, and caused obvious cell apoptosis and a loss of DeltaPsi(m) in RAW264.7 cells. Moreover, the ROS production in cells was elevated, and the levels of activated caspase-3 and caspase-9 were up-regulated in a dose-dependent manner. Notably, GA-induced cell apoptosis was markedly inhibited by caspase inhibitors. These results suggest that GA-induced RAW264.7 cell apoptosis may be mediated via a caspase-dependent mitochondrial signaling pathway.
MeSH Terms
-
Animals
Antineoplastic Agents, Phytogenic/*pharmacology
Apoptosis/*drug effects
Cell Line
Cell Proliferation/*drug effects
Dose-Response Relationship, Drug
Gossypol/*analogs & derivatives/pharmacology
Membrane Potential, Mitochondrial/*drug effects
Mice
Mice, Inbred BALB C
Reactive Oxygen Species/*metabolism
Signal Transduction/*drug effects
Antineoplastic Agents, Phytogenic
Gossypol
Reactive Oxygen Species
Figure
Reference
-
1. Borutaite V. Mitochondria as decision-makers in cell death. Environ Mol Mutagen. 2010; 51:406–416.
Article2. Cheng W, Li Y, Yang D, Zhao Y. Effect of gossypol acetate on proliferation and apoptosis in Raji lymphoblastoid cell line. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2009; 31:527–532.3. Chowdhury I, Tharakan B, Bhat GK. Current concepts in apoptosis: the physiological suicide program revisited. Cell Mol Biol Lett. 2006; 11:506–525.
Article4. Coutinho EM. Gossypol: a contraceptive for men. Contraception. 2002; 65:259–263.
Article5. Dao VT, Gaspard C, Mayer M, Werner GH, Nguyen SN, Michelot RJ. Synthesis and cytotoxicity of gossypol related compounds. Eur J Med Chem. 2000; 35:805–813.
Article6. Denault JB, Salvesen GS. Apoptotic caspase activation and activity. Methods Mol Biol. 2008; 414:191–220.
Article7. Deng S, Pawlak A, Poźniak B, Suszko A, Szczypka M, Yuan H, Obmińska-Mrukowicz B. Effects of gossypol acetic acid on cellular and humoral immune response in non-immunized and SRBC-immunized mice. Centr Eur J Immunol. 2012; 37:11–19.8. Dodou K, Anderson RJ, Small DA, Groundwater PW. Investigations on gossypol: past and present developments. Expert Opin Investig Drugs. 2005; 14:1419–1434.
Article9. Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999; 68:383–424.
Article10. Hass R, Pfannkuche HJ, Kharhanda S, Gunji H, Meyer G, Hartmann A, Hidaka H, Resch K, Kufe D, Goppelt-Strübe M. Protein kinase C activation and protooncogene expression in differentiation/retrodifferentiation of human U-937 leukemia cells. Cell Growth Differ. 1991; 2:541–548.11. He X, Zeng Y, Xu L, Sun H, Li Z, Di J. Influences of protein kinase C inhibitors on the expression of IL-2 and IFN-γ by human T-lymphocytes. Chin J Pathophysiol. 2002; 18:522–525.12. Hu CY, Jiang CH. The biological activities and mechanisms of gossypol. Foreign Med Sci. 1997; 16:68–72. (Family Planning/Reproductive Health Fascicle).13. Huang MM, Shao XX, Xiong ZH, Mao YE, Ouyang YG. The experimental research of taurine sodium gossypol immunosuppression. Acta Med Univ Sci Technol Huazhong. 1981; 10:77.14. Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997; 88:347–354.
Article15. Kindt TJ, Osborne BA, Goldsby RA. Kuby Immunology. 6th ed. New York: W.H. Freeman;2006. p. 37–44.16. Ko CH, Shen SC, Yang LY. Gossypol reduction of tumor growth through ROS-dependent mitochondria pathway in human colorectal carcinoma cells. Int J Cancer. 2007; 121:1670–1679.
Article17. Liang L, Wang SM, Hu XG, Cao MM, Zhang JR. Effects of gossypol acetic acid on CNE2 cells apoptosis. J Pract Med. 2008; 24:1009–1101.18. Lin HW, Chang TJ, Yang DJ, Chen YC, Wang M, Chang YY. Regulation of virus-induced inflammatory response by β-carotene in RAW264.7 cells. Food Chem. 2012; 134:2169–2175.
Article19. Long A, Wu J, Yuan L, Yuan H. Effect of gossypol on proliferation and apoptosis of primary luteal cells in sow. Chin J Vet Sci. 2009; 29:1303–1306.20. Lowenstein CJ, Dinerman JL, Snyder SH. Nitric oxide: a physiologic messenger. Ann Intern Med. 1994; 120:227–237.
Article21. Oliver CL, Miranda MB, Shangary S, Land S, Wang S, Johnson DE. (-)-Gossypol acts directly on the mitochondria to overcome Bcl-2- and Bcl-XL-mediated apoptosis resistance. Mol Cancer Ther. 2005; 4:23–31.22. Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007; 47:143–183.
Article23. Prasad MRN, Diczfalusy E. Gossypol. Int J Androl. 1982; 5:Suppl 5. 53–70.
Article24. Robinson JM, Tanphaichitr N, Bellvé AR. Gossypol-induced damage to mitochondria of transformed Sertoli cells. Am J Pathol. 1986; 125:484–492.25. Qian SZ, Wang ZG. Gossypol: a potential antifertility agent for males. Annu Rev Pharmacol Toxicol. 1984; 24:329–360.
Article26. Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000; 5:415–418.27. Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, Wang HG, Reed JC, Nicholson DW, Alnemri ES, Green DR, Martin SJ. Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2, -3, -6, -7, -8, and -10 in a caspase-9-dependent manner. J Cell Biol. 1999; 144:281–292.
Article28. Tiwari BS, Belenghi B, Levine A. Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol. 2002; 128:1271–1281.
Article29. Wang X, Howell CP, Chen F, Yin J, Jiang Y. Gossypol-A Polyphenolic Compound from Cotton Plant. Adv Food Nutr Res. 2009; 58:215–263.30. Wu J, Jing L, Yuan H, Peng S. T-2 toxin induces apoptosis in ovarian granulosa cells of rats through reactive oxygen species-mediated mitochondrial pathway. Toxicol Lett. 2011; 202:168–177.
Article31. Ye YX, Bombick D, Hirst K, Zhang GX, Chang CC, Trosko JE, Akera T. The modulation of gap junctional communication by gossypol in various mammalian cell lines in vitro. Fundam Appl Toxicol. 1990; 14:817–832.
Article32. Yurtcu E, Ergun MA, Menevse A. Apoptotic effect of gossypol on human lymphocytes. Cell Biol Int. 2003; 27:791–794.
Article33. Zhang M, Liu H, Tian Z, Griffith BN, Ji M, Li QQ. Gossypol induces apoptosis in human PC-3 prostate cancer cells by modulating caspase-dependent and caspaseindependent cell death pathways. Life Sci. 2007; 80:767–774.
Article34. Zhan WH, Yan G, Shao SP. Observation of apoptotic effect on human prostate cancer cell PC-3 and DU-145 induced by (-)-gossypol. Shandong Med. 2008; 48:86–87.35. Zheng YH, Wu ZH, Fang L. Effect of gossypol acetic acid on hCG-stimulated progesterone prodouction of dissociated rat luteal cells. Yao Xue Xue Bao. 1991; 26:805–808.36. Zhu L, Yuan H, Guo C, Lu Y, Deng S, Yang Y, Wei Q, Wen L, He Z. Zearalenone induces apoptosis and necrosis in porcine granulosa cells via a caspase-3- and caspase-9-dependent mitochondrial signaling pathway. J Cell Physiol. 2012; 227:1814–1820.
Article37. Zirk NM, Hashmi SF, Ziegler HK. The polysaccharide portion of lipopolysaccharide regulates antigen-specific T-cell activation via effects on macrophage-mediated antigen processing. Infect Immun. 1999; 67:319–326.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recombinant Rv0753c Protein of Mycobacterium tuberculosis Induces Apoptosis Through Reactive Oxygen Species-JNK Pathway in Macrophages
- Control of Mitochondrial Dynamics by Fas-induced Caspase-8 Activation in Hippocampal Neurons
- Quercetin induces cell death by caspase-dependent and p38 MAPK pathway in EGFR mutant lung cancer cells
- Paraquat Induces Apoptosis through a Mitochondria-Dependent Pathway in RAW264.7 Cells
- Arsenic Trioxide Induces Apoptosis of HL-60 Cells via Activation of Intrinsic Caspase Protease with Mitochondrial Dysfunction