J Bacteriol Virol.
2020 Dec;50(4):246-256. 10.4167/jbv.2020.50.4.246.
Recombinant Rv0753c Protein of Mycobacterium tuberculosis Induces Apoptosis Through Reactive Oxygen Species-JNK Pathway in Macrophages
- Affiliations
-
- 1Department of Microbiology and Medical Science, and Translational Immunology Institute, College of Medicine, Chungnam National University, Daejeon 35015, Republic of Korea
- KMID: 2512144
- DOI: http://doi.org/10.4167/jbv.2020.50.4.246
Abstract
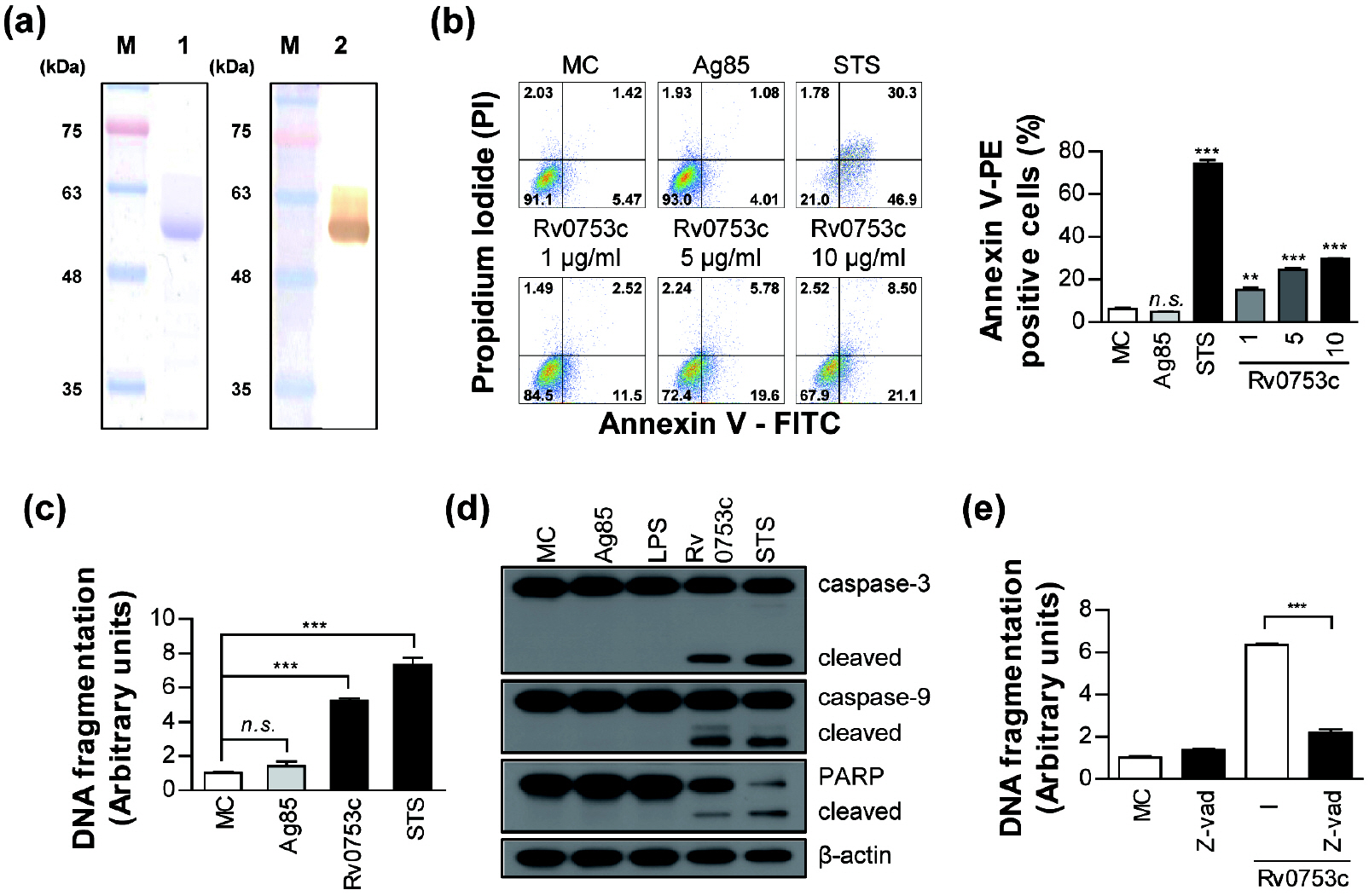
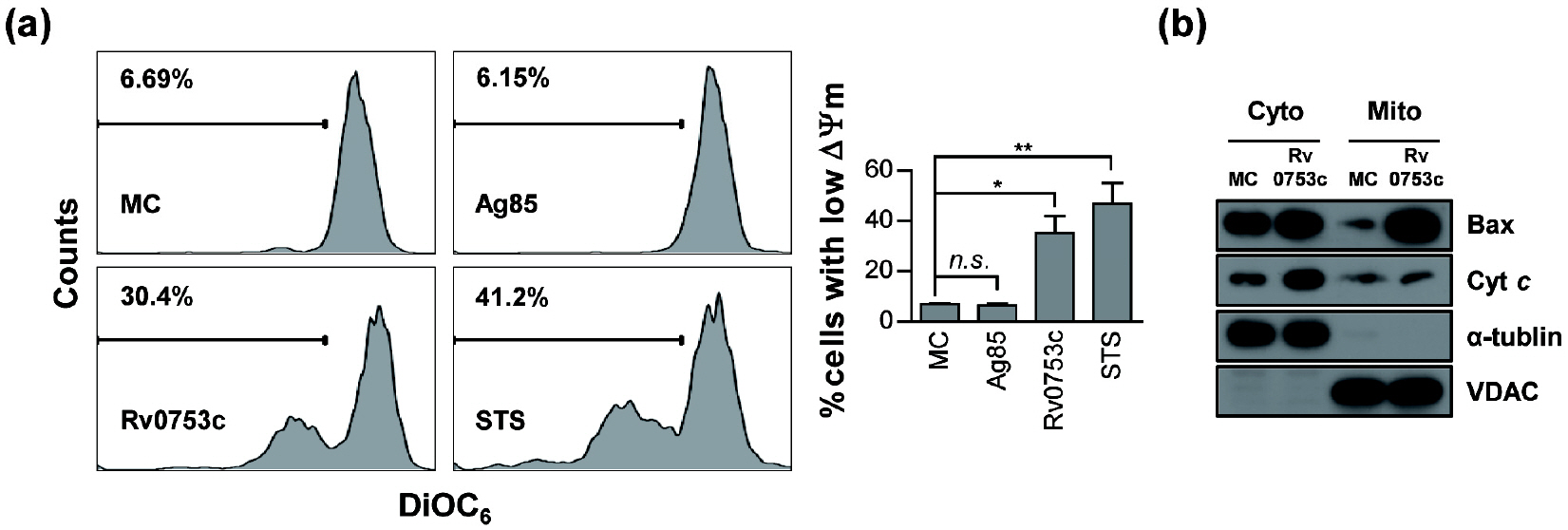
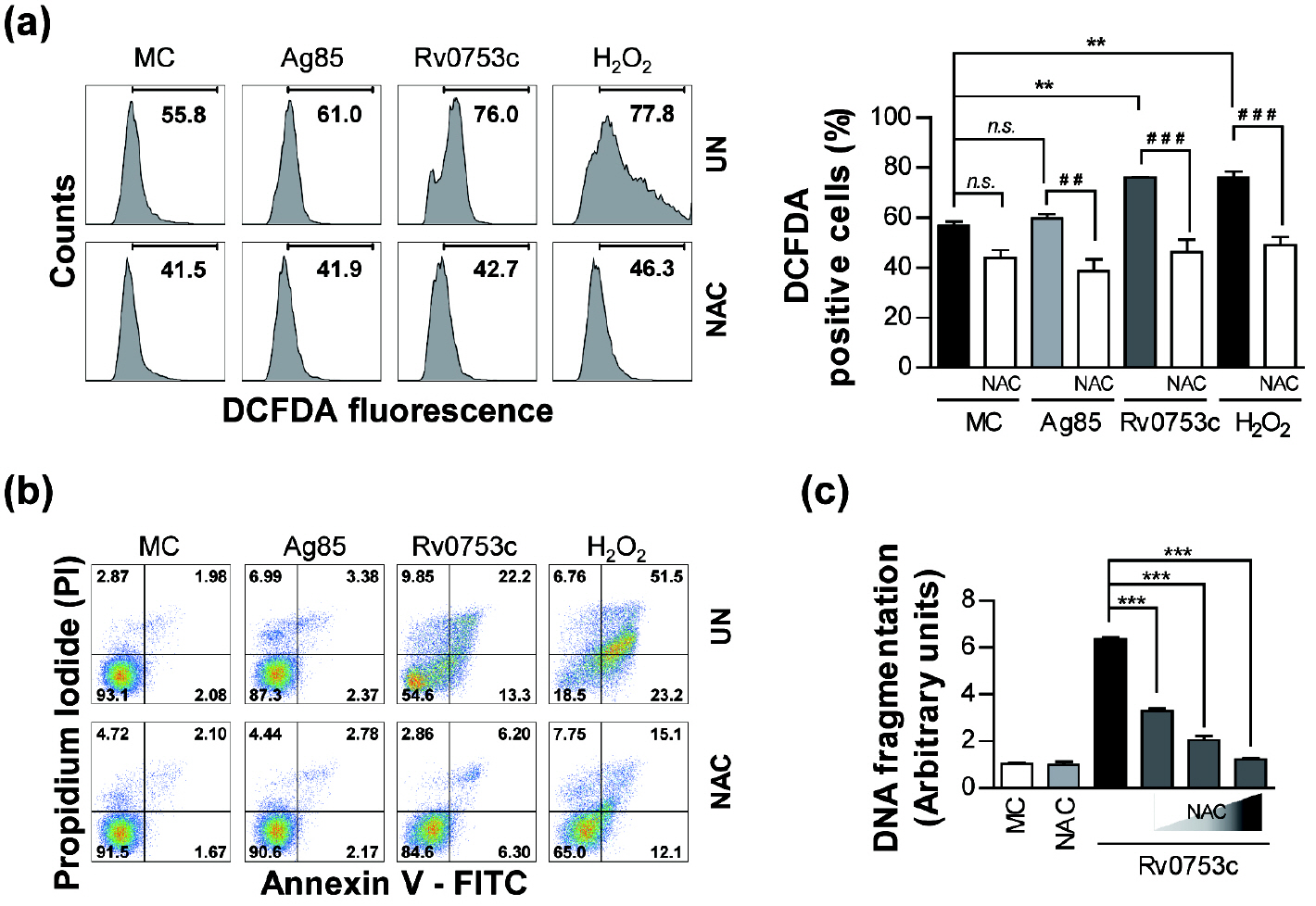
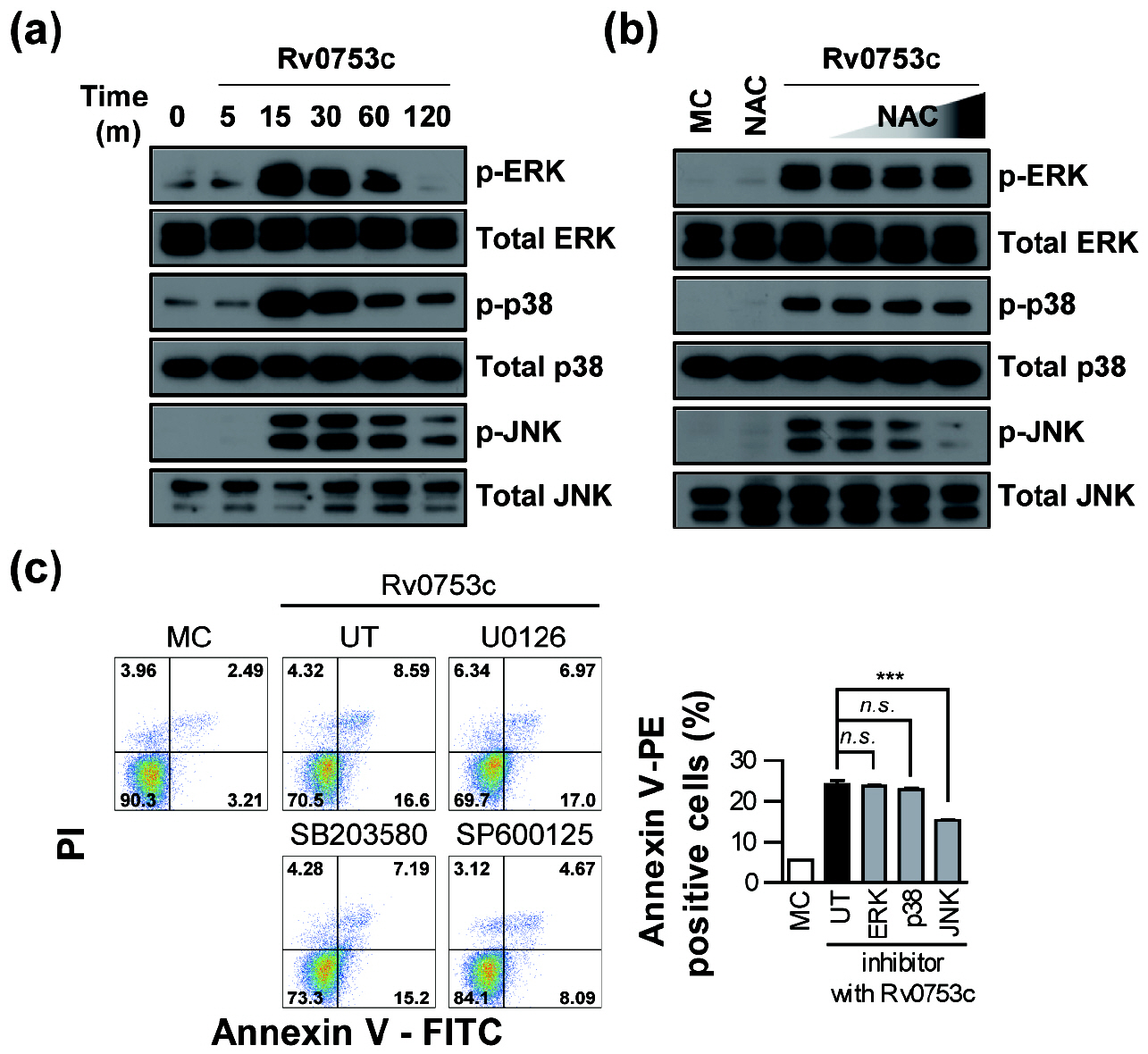
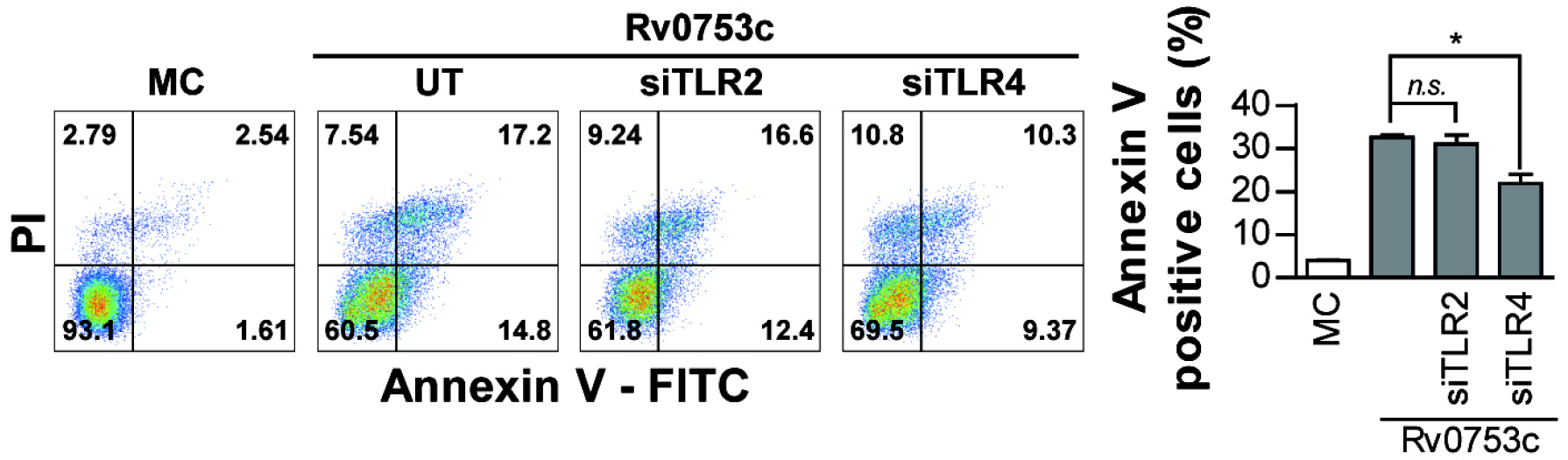
- Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), remains one of the most important infectious diseases worldwide. Mtb and its culture filtrates or sonic extracts induce apoptosis in macrophages. However, there is a little known about Mtb components that modulate apoptosis and their regulating mechanism. We identified Rv0753c protein with apoptotic potential through searching the biologic active proteins from the multidimensional fractions of Mtb culture filtrate. Here, we investigated the apoptotic effects of Rv0753c on RAW264.7 cells. The recombinant Rv0753c induced RAW264.7 cells apoptosis in a caspase-9-dependent manner. Dissipation of the mitochondrial transmembrane potential (ΔΨ m ), mitochondrial translocation of Bax, and release of cytochrome c from mitochondria were observed in macrophages treated with Rv0753c. Enhanced reactive oxygen species (ROS) production was required for Rv0753c-mediated apoptosis. Furthermore, ROS-mediated JNK activation was major signaling pathway for Rv0753c-induced apoptosis. Moreover, Rv0753c-mediated apoptosis is dependent on TLR4. Altogether, these results suggest that Rv0753c induce apoptosis through ROS-JNK signaling pathway in RAW264.7 cells.
Keyword
Figure
Reference
-
1. WHO guidelines on tuberculosis infection prevention and control: 2019 update. WHO Guidelines Approved by the Guidelines Review Committee. Geneva 2019.2. Park HS, Back YW, Shin KW, Bae HS, Lee KI, Choi HG, et al. Mycobacterium tuberculosis Rv3463 induces mycobactericidal activity in macrophages by enhancing phagolysosomal fusion and exhibits therapeutic potential. Sci Rep 2019;9:4246.DOI: 10.1038/s41598-019-38982-0. PMID: 30862819. PMCID: PMC6414722.3. Guo Y, Deng Y, Huang Z, Luo Q, Peng Y, Chen J, et al. EBP50 induces apoptosis in macrophages by upregulating nitric oxide production to eliminate intracellular Mycobacterium tuberculosis. Sci Rep 2016;6:18961.DOI: 10.1038/srep18961. PMID: 26729618. PMCID: PMC4700441.4. Meng CY, Liang X, Wei K, Yang YR. Enantioselective Ir-Catalyzed Allylic Alkylation of Racemic Allylic Alcohols with Malonates. Org Lett 2019;21:840-3.DOI: 10.1021/acs.orglett.8b04143. PMID: 30673252.5. Parandhaman DK, Narayanan S. Cell death paradigms in the pathogenesis of Mycobacterium tuberculosis infection. Front Cell Infect Microbiol 2014;4:31.DOI: 10.3389/fcimb.2014.00031. PMID: 24634891. PMCID: PMC3943388.6. Aslan M, Ozben T. Oxidants in receptor tyrosine kinase signal transduction pathways. Antioxid Redox Signal 2003;5:781-8.DOI: 10.1089/152308603770380089. PMID: 14588151.7. Sohn H, Kim JS, Shin SJ, Kim K, Won CJ, Kim WS, et al. Targeting of Mycobacterium tuberculosis heparin-binding hemagglutinin to mitochondria in macrophages. PLoS Pathog 2011;7:e1002435.DOI: 10.1371/journal.ppat.1002435. PMID: 22174691. PMCID: PMC3234249.8. Lee KI, Whang J, Choi HG, Son YJ, Jeon HS, Back YW, et al. Mycobacterium avium MAV2054 protein induces macrophage apoptosis by targeting mitochondria and reduces intracellular bacterial growth. Sc Rep 2016;6:37804.DOI: 10.1038/srep37804. PMID: 27901051. PMCID: PMC5129020.9. Butler RE, Brodin P, Jang J, Jang MS, Robertson BD, Gicquel B, et al. The balance of apoptotic and necrotic cell death in Mycobacterium tuberculosis infected macrophages is not dependent on bacterial virulence. PloS one 2012;7:e47573.DOI: 10.1371/journal.pone.0047573. PMID: 23118880. PMCID: PMC3484146.10. Fratazzi C, Arbeit RD, Carini C, Balcewicz-Sablinska MK, Keane J, Kornfeld H, et al. Macrophage apoptosis in mycobacterial infections. J Leukoc Biol 1999;66:763-4.DOI: 10.1002/jlb.66.5.763. PMID: 10577507.11. Zhang J, Jiang R, Takayama H, Tanaka Y. Survival of virulent Mycobacterium tuberculosis involves preventing apoptosis induced by Bcl-2 upregulation and release resulting from necrosis in J774 macrophages. Microbiol Immunol 2005;49:845-52.DOI: 10.1111/j.1348-0421.2005.tb03673.x. PMID: 16172539.12. Awuh JA, Flo TH. Author Correction: Molecular basis of mycobacterial survival in macrophages. Cell Mol Life Sci 2018;75:161.DOI: 10.1007/s00018-017-2683-x. PMID: 29022045.13. Lin J, Chang Q, Dai X, Liu D, Jiang Y, Dai Y. Early secreted antigenic target of 6-kDa of Mycobacterium tuberculosis promotes caspase-9/caspase-3-mediated apoptosis in macrophages. Mol Cell Biochem 2019;457:179-89.DOI: 10.1007/s11010-019-03522-x. PMID: 30911956.14. Sánchez A, Espinosa P, García T, Mancilla R. The 19 kDa Mycobacterium tuberculosis lipoprotein (LpqH) induces macrophage apoptosis through extrinsic and intrinsic pathways: a role for the mitochondrial apoptosis-inducing factor. Clin Dev Immunol 2012;2012:950503.DOI: 10.1155/2012/950503. PMID: 23316255. PMCID: PMC3536062.15. Lee KI, Choi S, Choi HG, Kebede SG, Dang TB, Back YW, et al. Recombinant Rv3261 protein of Mycobacterium tuberculosis induces apoptosis through a mitochondrion-dependent pathway in macrophages and inhibits intracellular bacterial growth. Cell Immunol 2020;354:104145.DOI: 10.1016/j.cellimm.2020.104145. PMID: 32569876.16. Lee KI, Choi HG, Son YJ, Whang J, Kim K, Jeon HS, et al. Mycobacterium avium MAV2052 protein induces apoptosis in murine macrophage cells through Toll-like receptor 4. Apoptosis 2016;21:459-72.DOI: 10.1007/s10495-016-1220-y. PMID: 26842846.17. Choi HG, Choi S, Back YW, Park HS, Bae HS, Choi CH, et al. Mycobacterium tuberculosis Rv2882c Protein Induces Activation of Macrophages through TLR4 and Exhibits Vaccine Potential. PloS One 2016;11:e0164458.DOI: 10.1371/journal.pone.0164458. PMID: 27711141. PMCID: PMC5053528.18. Sohn H, Kim K, Lee KS, Choi HG, Lee KI, Shin AR, et al. Lithium inhibits growth of intracellular Mycobacterium kansasii through enhancement of macrophage apoptosis. J Microbiol 2014;52:299-306.DOI: 10.1007/s12275-014-3469-6. PMID: 24535745.19. Paik S, Choi S, Lee KI, Back YW, Son YJ, Jo EK, et al. Mycobacterium tuberculosis acyl carrier protein inhibits macrophage apoptotic death by modulating the reactive oxygen species/c-Jun N-terminal kinase pathway. Microbes Infect 2019;21:40-9.DOI: 10.1016/j.micinf.2018.06.005. PMID: 29981934.20. Whang J, Back YW, Lee KI, Fujiwara N, Paik S, Choi CH, et al. Mycobacterium abscessus glycopeptidolipids inhibit macrophage apoptosis and bacterial spreading by targeting mitochondrial cyclophilin D. Cell Death Dis 2017;8:e3012.DOI: 10.1038/cddis.2017.420. PMID: 28837151. PMCID: PMC5596598.21. Choi S, Choi HG, Shin KW, Back YW, Park HS, Lee JH, et al. Mycobacterium tuberculosis Protein Rv3841 Activates Dendritic Cells and Contributes to a T Helper 1 Immune Response. J Immunol Res 2018;2018:3525302.DOI: 10.1155/2018/3525302. PMID: 29736404. PMCID: PMC5875036.22. Kroemer G. Mitochondrial control of apoptosis: an overview. Biochem Soc Symp 1999;66:1-15.DOI: 10.1042/bss0660001. PMID: 10989652.23. Lim YJ, Choi HH, Choi JA, Jeong JA, Cho SN, Lee JH, et al. Mycobacterium kansasii-induced death of murine macrophages involves endoplasmic reticulum stress responses mediated by reactive oxygen species generation or calpain activation. Apoptosis 2013;18:150-9.DOI: 10.1007/s10495-012-0792-4. PMID: 23264129.24. Azad N, Iyer AKV, Manosroi A, Wang L, Rojanasakul Y. Superoxide-mediated proteasomal degradation of Bcl-2 determines cell susceptibility to Cr(VI)-induced apoptosis. Carcinogenesis 2008;29:1538-45.DOI: 10.1093/carcin/bgn137. PMID: 18544562. PMCID: PMC2516494.25. López M, Sly LM, Luu Y, Young D, Cooper H, Reiner NE. The 19-kDa Mycobacterium tuberculosis protein induces macrophage apoptosis through Toll-like receptor-2. J Immunol 2003;170:2409-16.DOI: 10.4049/jimmunol.170.5.2409. PMID: 12594264.26. Grover S, Sharma T, Singh Y, Kohli S, P M, Singh A, et al. The PGRS Domain of Mycobacterium tuberculosis PE_PGRS Protein Rv0297 Is Involved in Endoplasmic Reticulum Stress-Mediated Apoptosis through Toll-Like Receptor 4. mBio 2018;9:e01017-8.DOI: 10.1128/mBio.01017-18. PMID: 29921671. PMCID: PMC6016250.27. Rodrigues MF, Barsante MM, Alves CCS, Souza MA, Ferreira AP, Amarante-Mendes GP, et al. Apoptosis of macrophages during pulmonary Mycobacterium bovis infection: correlation with intracellular bacillary load and cytokine levels. Immunology 2009;128:e691-9.DOI: 10.1111/j.1365-2567.2009.03062.x. PMID: 19740330. PMCID: PMC2753962.28. Mahamed D, Boulle M, Ganga Y, Mc Arthur C, Skroch S, Oom L, et al. Intracellular growth of Mycobacterium tuberculosis after macrophage cell death leads to serial killing of host cells. Elife 2017;6:e22028.DOI: 10.7554/eLife.28205. PMID: 28475039. PMCID: PMC5419738.29. Kim JS, Kim WS, Choi HH, Kim HM, Kwon KW, Han SJ, et al. Mycobacterium tuberculosis MmsA, a novel immunostimulatory antigen, induces dendritic cell activation and promotes Th1 cell-type immune responses. Cell Immunol 2015;298:115-25.DOI: 10.1016/j.cellimm.2015.10.005. PMID: 26507911.30. Pathakumari B, Devasundaram S, Maddineni P, Raja A. Rv2204c, Rv0753c and Rv0009 antigens specific T cell responses in latent and active TB - a flow cytometry-based analysis. Int J Med Microbiol 2018;308:297-305.DOI: 10.1016/j.ijmm.2017.12.001. PMID: 29325881.31. Green DR, Reed JC. Mitochondria and apoptosis. Science 1998;281:1309-12.DOI: 10.1126/science.281.5381.1309. PMID: 9721092.32. Fleury C, Mignotte B, Vayssière JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie 2002;84:131-41.DOI: 10.1016/S0300-9084(02)01369-X.33. Kim K, Sohn H, Kim JS, Choi HG, Byun EH, Lee KI, et al. Mycobacterium tuberculosis Rv0652 stimulates production of tumour necrosis factor and monocytes chemoattractant protein-1 in macrophages through the Toll-like receptor 4 pathway. Immunology 2012;136:231-40.DOI: 10.1111/j.1365-2567.2012.03575.x. PMID: 22385341. PMCID: PMC3403266.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intracellular Signaling Pathways that Regulate Macrophage Chemokine Expression in Response to Mycobacterium abscessus
- Paraquat Induces Apoptosis through Cytochrome C Release and ERK Activation
- Apoptotic Effect of Macrophages against Mycobacterium tuberculosis
- Novel Therapeutic Target of Non-alcoholic Fatty Liver Disease: Nicotinamide Adenine Dinucleotide Phosphate Oxidase 2 and Reactive Oxygen Species in Pro-inflammatory Macrophages
- YJI-7 Suppresses ROS Production and Expression of Inflammatory Mediators via Modulation of p38MAPK and JNK Signaling in RAW 264.7 Macrophages