Korean J Hematol.
2005 Sep;40(3):142-148. 10.5045/kjh.2005.40.3.142.
Hematologic Recovery and Clinical Outcomes according to Cell Dose after HLA-matched Sibling Allogeneic Bone Marrow Transplantation
- Affiliations
-
- 1Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea. kimhj@dau.ac.kr
- 2Department of Laboratory Medicine, Dong-A University College of Medicine, Busan, Korea.
- KMID: 1610807
- DOI: http://doi.org/10.5045/kjh.2005.40.3.142
Abstract
- BACKGROUND
There has been changed in estimation of the stem cell content of the graft for several decades. However, there is not always correlating the transplanted cell dose with hematologic recovery, and there are few reports in human leukocyte antigen (HLA)-matched sibling allogeneic bone marrow transplantation (AlloBMT) in Korea. The purpose of this study is to report the influence of number of transplanted cell dose on hematologic recovery and the clinical outcomes in HLA-matched sibling AlloBMT.
METHODS
Between June 1999 and March 2004, 31 AlloBMT from HLA-matched sibling donor was done in patients with hematologic malignancy. All patients were conditioned with busulfan and cyclophosphamide. Short course methotrexate and cyclosporine regimen was used for prophylaxis of graft-versus-host disease. We analyzed hematologic recovery time and clinical outcomes according to transplanted cell dose.
RESULTS
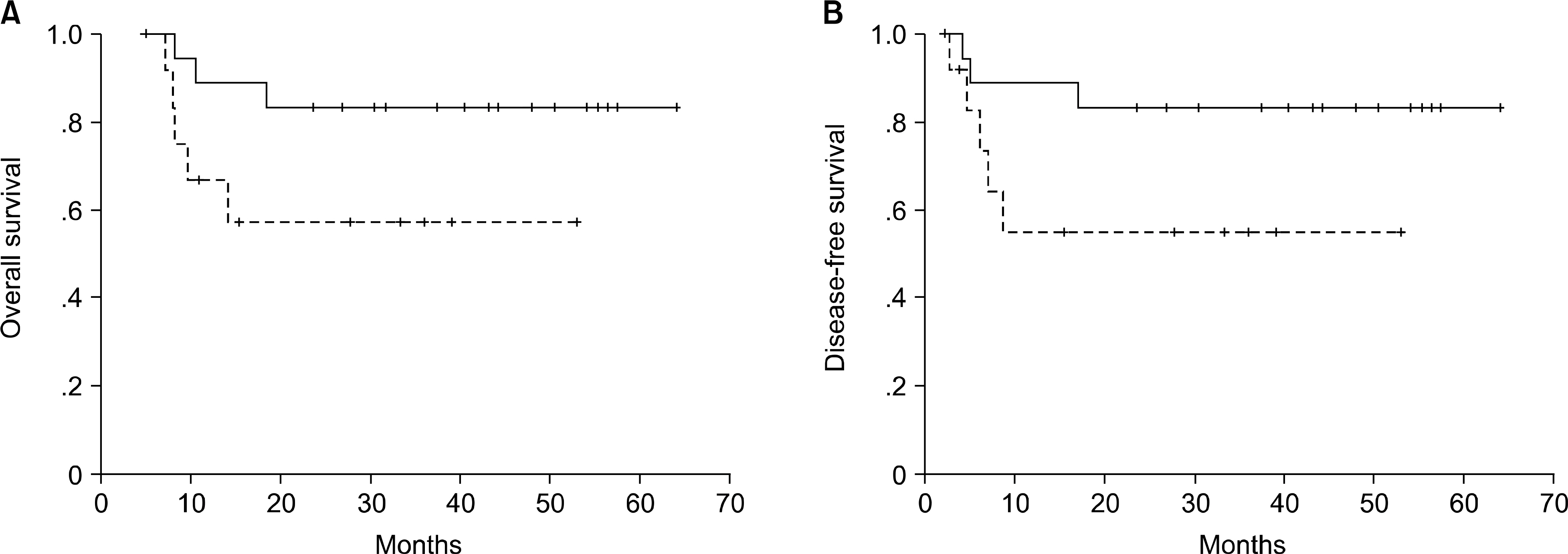
There were 16 male and 15 female patients, with a median age of 34 years (range, 16~48). Underlying diseases were 17 acute myeloid leukemia, 4 acute lymphoblastic leukemia, 3 myelodysplastic syndrome (high-risk), and 7 chronic myelogenous leukemia. The median number of total nucleated cell (TNC), mononuclear cell (MNC) and CD34+ cell infused was 3.95x10(8)/kg (range, 1.67~7.30x10(8)/kg), 0.65x10(8)/kg (range, 0.11~2.50x10(8)/kg), and 2.32x106/kg (range, 0.35~7.45x106/kg), respectively. The median days of neutrophil and platelet engraftment (ANC>500/microliter and platelet > 20,000/L without transfusion) were 15 (range, 10~19), 16 (range, 7~37), respectively. Relationship between the rate of neutrophil engraftment and the number of infused TNC was only statistically significant (P=0.038, R2=0.328). This study showed survival benefit with the increment of CD34+ cell dose without significance statistically (P=0.082).
CONCLUSION
Although the dose of the number of transplanted MNC and CD34+ cells had no influence on granulocyte or platelet recovery, the number of TNC had only a beneficial effect on neutrophil recovery. The transplanted dose of CD34+ cells, rather than those of TNC and MNC may be related with better survival.
MeSH Terms
-
Blood Platelets
Bone Marrow Transplantation*
Bone Marrow*
Busulfan
Cyclophosphamide
Cyclosporine
Female
Graft vs Host Disease
Granulocytes
Hematologic Neoplasms
Humans
Korea
Leukemia, Myelogenous, Chronic, BCR-ABL Positive
Leukemia, Myeloid, Acute
Leukocytes
Male
Methotrexate
Myelodysplastic Syndromes
Neutrophils
Precursor Cell Lymphoblastic Leukemia-Lymphoma
Siblings*
Stem Cells
Tissue Donors
Transplants
Busulfan
Cyclophosphamide
Cyclosporine
Methotrexate
Figure
Reference
-
1). Atkinson K, Norrie S, Chan P, Downs K, Biggs J. Lack of correlation between nucleated bone marrow cell dose, marrow CFU-GM dose or marrow CFU-E dose and the rate of HLA-identical sibling marrow engraftment. Br J Haematol. 1985; 60:245–51.
Article2). Arnold R, Schmeiser T, Heit W, et al. Hemopoietic reconstitution after bone marrow transplantation. Exp Hematol. 1986; 14:271–7.3). Haas R, Witt B, Mohle R, et al. Sustained longterm hematopoiesis after myeloablative therapy with peripheral blood progenitor cell support. Blood. 1995; 85:3754–61.
Article4). Mavroudis D, Read E, Cottler-Fox M, et al. CD34+ cell dose predicts survival, posttransplant morbidity, and rate of hematologic recovery after allogeneic marrow transplants for hematologic malignancies. Blood. 1996; 88:3223–9.
Article5). Nakamura R, Bahceci E, Read EJ, et al. Transplant dose of CD34 (+) and CD3 (+) cells predicts outcome in patients with haematological malignancies undergoing T cell-depleted peripheral blood stem cell transplants with delayed donor lymphocyte add-back. Br J Haematol. 2001; 115:95–104.6). Urbano-Ispizua A, Carreras E, Marin P, et al. Allogeneic transplantation of CD34 (+) selected cells from peripheral blood from human leukocyte antigen-identical siblings: detrimental effect of a high number of donor CD34 (+) cells? Blood. 2001; 98:2352–7.7). Dominietto A, Raiola AM, van Lint MT, et al. Factors influencing haematological recovery after allogeneic haemopoietic stem cell transplants: graft-versus-host disease, donor type, cytomegalovirus infections and cell dose. Br J Haematol. 2001; 112:219–27.
Article8). Chae YS, Jeon SB, Sung WJ, et al. Clinical outcomes according to transplanted CD34+cell dose in allogeneic peripheral blood stem cell transplantation. Korean J Hematol. 2003; 38:24–31.9). Thomas ED, Storb R, Clift RA, et al. Bone-marrow transplantation. N Engl J Med. 1975; 292:895–902.
Article10). Torres A, Alonso MC, Gomez-Villagran JL, et al. No influence of number of donor CFU-GM on granulocyte recovery in bone marrow transplantation for acute leukemia. Blut. 1985; 50:89–94.
Article11). To LB, Roberts MM, Haylock DN, et al. Comparison of haematological recovery times and supportive care requirements of autologous recovery phase peripheral blood stem cell transplants, autologous bone marrow transplants and allogeneic bone marrow transplants. Bone Marrow Transplant. 1992; 9:277–84.12). Sierra J, Storer B, Hansen JA, et al. Transplantation of marrow cells from unrelated donors for treatment of high-risk acute leukemia: the effect of leukemic burden, donor HLA-matching, and marrow cell dose. Blood. 1997; 89:4226–35.
Article13). Dominietto A, Lamparelli T, Raiola AM, et al. Transplant-related mortality and longterm graft function are significantly influenced by cell dose in patients undergoing allogeneic marrow transplantation. Blood. 2002; 100:3930–4.
Article14). Bensinger WI, Longin K, Appelbaum FR, et al. Peripheral blood stem cells (PBSCs) collected after recombinant granulocyte colony stimulating factor (rhG-CSF): an analysis of factors correlating with the tempo of engraftment after transplantation. Br J Haematol. 1994; 87:825–31.
Article15). Bahceci E, Read EJ, Leitman S, et al. CD34+ cell dose predicts relapse and survival after T-cell-depleted HLA-identical haematopoietic stem cell transplantation (HSCT) for haematological malignancies. Br J Haematol. 2000; 108:408–14.16). Morariu-Zamfir R, Rocha V, Devergie A, et al. Influence of CD34 (+) marrow cell dose on outcome of HLA-identical sibling allogeneic bone marrow transplants in patients with chronic myeloid leukaemia. Bone Marrow Transplant. 2001; 27:575–80.17). Bensinger WI, Clift R, Martin P, et al. Allogeneic peripheral blood stem cell transplantation in patients with advanced hematologic malignancies: a retrospective comparison with marrow transplantation. Blood. 1996; 88:2794–800.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pediatric Allogeneic Hematopoietic Stem Cell Transplantation in Korea: April 2000: The Korean Society of Pediatric Hematology-Oncology
- Reconstitution of Bone Marrow after Allogeneic and Autologous Bone Marrow Transplantation
- Successful Selective CD34+ Cell Infusion after Late Graft Failure of Hematopoietic Stem Cell Transplantation in Two Cases of Severe Aplastic Anemia
- Allogeneic Bone Marrow Transplantation Experience for Children with Severe Aplastic Anemia and Refractory Leukemia
- A Case of Rh-incompatible Allogeneic Bone Marrow Transplantation: Transplant of E- and c-positive Bone Marrow in a Recipient with Anti-E and Anti-c Antibodies