J Korean Surg Soc.
2013 Jun;84(6):313-320. 10.4174/jkss.2013.84.6.313.
Correlation between the molecular subtype of breast cancer and the in vitro adenosine triphosphate-based chemosensitivity assay
- Affiliations
-
- 1Department of Surgery, Ewha Womans University School of Medicine, Seoul, Korea. mbit@ewha.ac.kr
- 2Department of Pathology, Ewha Womans University School of Medicine, Seoul, Korea.
- KMID: 1505180
- DOI: http://doi.org/10.4174/jkss.2013.84.6.313
Abstract
- PURPOSE
The empirical use of a chemotherapy regimen shows different results in individual breast cancer patient treatment. Recent studies showed the effectiveness of the adenosine triphosphate-based chemotherapy response assay (ATP-CRA). However, little is known about the correlation between chemosensitivity and breast cancer molecular subtypes. Therefore, we investigated whether the result of ATP-CRA is associated with a molecular subtype of breast cancer.
METHODS
Two hundred eighty-seven patients diagnosed with breast cancer and receiving ATP-CRA at Mokdong Hospital, Ewha Womans University between September 2007 and December 2010 were enrolled in this study. Hormone receptor status, HER2/neu expression, and results of chemosensitivity tests of the patients was analyzed.
RESULTS
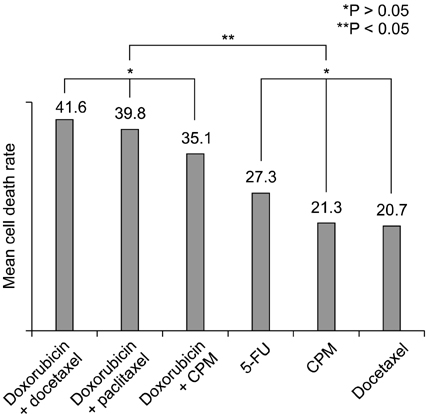
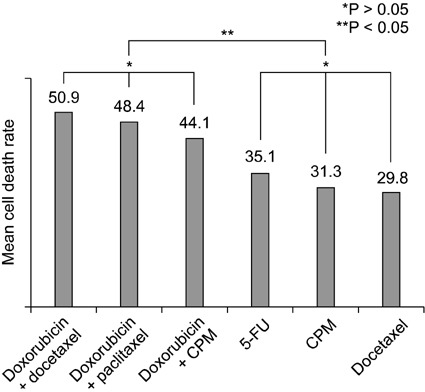
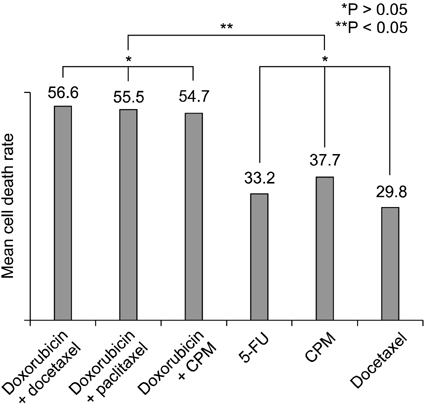
In all of four subtypes, the combination of two agents showed significant higher mean cell death rate than a single agent. Within the breast cancer cell lines in this study, the range of chemosensitivity response was very wide and varied for each patient. For this reason, the molecular subtype of breast cancer is inconclusive in choosing an effective chemotherapeutic agent and in vitro chemosensitivity test, prior to therapy, could be a useful method for planning chemotherapy for each patient.
CONCLUSION
Chemosensitivity response to anticancer agents was found to vary depending on the individual breast cancer patients. The molecular subtype of breast cancer is inconclusive to choose the effective chemotherapeutic agent and the in vitro chemosensitivity test, prior to therapy, could be more useful for planning chemotherapy for each patient.
MeSH Terms
Figure
Reference
-
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.2. Ministry of Health and Welfare, Korea Central Cancer Registry, National Cancer Center. Annual report of cancer statistics in Korea in 2008. Goyang: Ministry of Health and Welfare, Korea Central Cancer Registry, National Cancer Center;2010.3. Takamura Y, Kobayashi H, Taguchi T, Motomura K, Inaji H, Noguchi S. Prediction of chemotherapeutic response by collagen gel droplet embedded culture-drug sensitivity test in human breast cancers. Int J Cancer. 2002; 98:450–455.4. Kern DH, Drogemuller CR, Kennedy MC, Hildebrand-Zanki SU, Tanigawa N, Sondak VK. Development of a miniaturized, improved nucleic acid precursor incorporation assay for chemosensitivity testing of human solid tumors. Cancer Res. 1985; 45(11 Pt 1):5436–5441.5. Tanigawa N, Morimoto H, Dohmae N, Shimomatsuya T, Takahashi K, Muraoka R. In vitro growth ability and chemosensitivity of gastric and colorectal cancer cells assessed with the human tumour clonogenic assay and the thymidine incorporation assay. Eur J Cancer. 1992; 28:31–34.6. Wilbur DW, Camacho ES, Hilliard DA, Dill PL, Weisenthal LM. Chemotherapy of non-small cell lung carcinoma guided by an in vitro drug resistance assay measuring total tumour cell kill. Br J Cancer. 1992; 65:27–32.7. Mechetner E, Kyshtoobayeva A, Zonis S, Kim H, Stroup R, Garcia R, et al. Levels of multidrug resistance (MDR1) P-glycoprotein expression by human breast cancer correlate with in vitro resistance to taxol and doxorubicin. Clin Cancer Res. 1998; 4:389–398.8. Furukawa T, Kubota T, Hoffman RM. Clinical applications of the histoculture drug response assay. Clin Cancer Res. 1995; 1:305–311.9. Sevin BU, Peng ZL, Perras JP, Ganjei P, Penalver M, Averette HE. Application of an ATP-bioluminescence assay in human tumor chemosensitivity testing. Gynecol Oncol. 1988; 31:191–204.10. Andreotti PE, Cree IA, Kurbacher CM, Hartmann DM, Linder D, Harel G, et al. Chemosensitivity testing of human tumors using a microplate adenosine triphosphate luminescence assay: clinical correlation for cisplatin resistance of ovarian carcinoma. Cancer Res. 1995; 55:5276–5282.11. Konecny G, Crohns C, Pegram M, Felber M, Lude S, Kurbacher C, et al. Correlation of drug response with the ATP tumorchemosensitivity assay in primary FIGO stage III ovarian cancer. Gynecol Oncol. 2000; 77:258–263.12. Kim HA, Yom CK, Moon BI, Choe KJ, Sung SH, Han WS, et al. The use of an in vitro adenosine triphosphate-based chemotherapy response assay to predict chemotherapeutic response in breast cancer. Breast. 2008; 17:19–26.13. O'Meara AT, Sevin BU. Predictive value of the ATP chemosensitivity assay in epithelial ovarian cancer. Gynecol Oncol. 2001; 83:334–342.14. Moon YW, Sohn JH, Kim YT, Chang H, Jeong JH, Lee YJ, et al. Adenosine triphosphate-based chemotherapy response assay (ATP-CRA)-guided versus empirical chemotherapy in unresectable non-small cell lung cancer. Anticancer Res. 2009; 29:4243–4249.15. Kawamura H, Ikeda K, Takiyama I, Terashima M. The usefulness of the ATP assay with serum-free culture for chemosensitivity testing of gastrointestinal cancer. Eur J Cancer. 1997; 33:960–966.16. Huh JW, Park YA, Sohn SK, Choi SH. In-vitro Chemosensitivity test for colorectal cancer using an adenosine-triphosphate-based chemotherapy response assay (ATP-CRA). J Korean Soc Coloproctol. 2007; 23:172–179.17. Allred DC, Clark GM, Tandon AK, Molina R, Tormey DC, Osborne CK, et al. HER-2/neu in node-negative breast cancer: prognostic significance of overexpression influenced by the presence of in situ carcinoma. J Clin Oncol. 1992; 10:599–605.18. Maehara Y, Anai H, Tamada R, Sugimachi K. The ATP assay is more sensitive than the succinate dehydrogenase inhibition test for predicting cell viability. Eur J Cancer Clin Oncol. 1987; 23:273–276.19. Petty RD, Sutherland LA, Hunter EM, Cree IA. Comparison of MTT and ATP-based assays for the measurement of viable cell number. J Biolumin Chemilumin. 1995; 10:29–34.20. Woo SU, Bae JW, Kim HG, Choi SH, Kang DH, Lee JB, et al. Correlation between the in vitro ATP-based chemosensitivity assay and HER2/neu expression in women with breast cancer. J Int Med Res. 2007; 35:753–761.21. Choi SK, Jeong J, Lee SA, Hwang SH, Ahn SG, Jung WH, et al. Heterogeneous chemosensitivity of breast cancer determined by adeonsine triphosphate based chemotherapy response assay. J Breast Cancer. 2010; 13:180–186.22. Charfare H, Limongelli S, Purushotham AD. Neoadjuvant chemotherapy in breast cancer. Br J Surg. 2005; 92:14–23.23. Ogston KN, Miller ID, Schofield AC, Spyrantis A, Pavlidou E, Sarkar TK, et al. Can patients' likelihood of benefiting from primary chemotherapy for breast cancer be predicted before commencement of treatment? Breast Cancer Res Treat. 2004; 86:181–189.24. Hannemann J, Oosterkamp HM, Bosch CA, Velds A, Wessels LF, Loo C, et al. Changes in gene expression associated with response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2005; 23:3331–3342.25. Pritchard KI, Shepherd LE, O'Malley FP, Andrulis IL, Tu D, Bramwell VH, et al. HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med. 2006; 354:2103–2111.26. Rozan S, Vincent-Salomon A, Zafrani B, Validire P, De Cremoux P, Bernoux A, et al. No significant predictive value of c-erbB-2 or p53 expression regarding sensitivity to primary chemotherapy or radiotherapy in breast cancer. Int J Cancer. 1998; 79:27–33.27. Spitale A, Mazzola P, Soldini D, Mazzucchelli L, Bordoni A. Breast cancer classification according to immunohistochemical markers: clinicopathologic features and short-term survival analysis in a population-based study from the South of Switzerland. Ann Oncol. 2009; 20:628–635.28. Zaha DC, Lazar E, Lazureanu C. Clinicopathologic features and five years survival analysis in molecular subtypes of breast cancer. Rom J Morphol Embryol. 2010; 51:85–89.29. Chen XS, Wu JY, Huang O, Chen CM, Wu J, Lu JS, et al. Molecular subtype can predict the response and outcome of Chinese locally advanced breast cancer patients treated with preoperative therapy. Oncol Rep. 2010; 23:1213–1220.30. Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005; 11:5678–5685.31. Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007; 13:2329–2334.32. Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001; 98:10869–10874.33. Gianni L, Zambetti M, Clark K, Baker J, Cronin M, Wu J, et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J Clin Oncol. 2005; 23:7265–7277.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Heterogeneous Chemosensitivity of Breast Cancer Determined by Adeonsine Triphosphate Based Chemotherapy Response Assay
- Correlation of Early Systemic Recurrence with In Vitro Adenosine Triphosphate-Based Chemotherapy Response Assay in Stage II and III Breast Cancer Patients Treated with Doxorubicin-Based Chemotherapy
- In Vitro Adenosine Triphosphate-Based Chemotherapy Response Assay as a Predictor of Clinical Response to Fluorouracil-Based Adjuvant Chemotherapy in Stage II Colorectal Cancer
- In Vitro Adenosine Triphosphate Based Chemotherapy Response Assay in Gastric Cancer
- In-vitro Chemosensitivity Test for Colorectal Cancer using an Adenosine-triphosphate-based Chemotherapy Response Assay (ATP-CRA)