J Vet Sci.
2013 Mar;14(1):69-76. 10.4142/jvs.2013.14.1.69.
Survival of hypoxic human mesenchymal stem cells is enhanced by a positive feedback loop involving miR-210 and hypoxia-inducible factor 1
- Affiliations
-
- 1Department of Biology Education, College of Education, Pusan National University, Busan 609-735, Korea.
- 2Institute of Catholic Integrative Medicine, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Incheon 403-720, Korea.
- 3Cardiovascular Research Institute, Yonsei University College of Medicine, Seoul 120-752, Korea. kchwang@yuhs.ac
- 4Brain Korea 21 Project for Medical Science, Yonsei University College of Medicine, Seoul 120-752, Korea.
- 5Severance Biomedical Science Institute, Yonsei University College of Medicine, Seoul 120-752, Korea.
- 6Department of Integrated Omics for Biomedical Sciences, Graduate School, Yonsei University, Seoul 120-749, Korea.
- 7Department of Molecular Physiology, College of Pharmacy, Kyungpook National University, Daegu 702-701, Korea.
- KMID: 1482815
- DOI: http://doi.org/10.4142/jvs.2013.14.1.69
Abstract
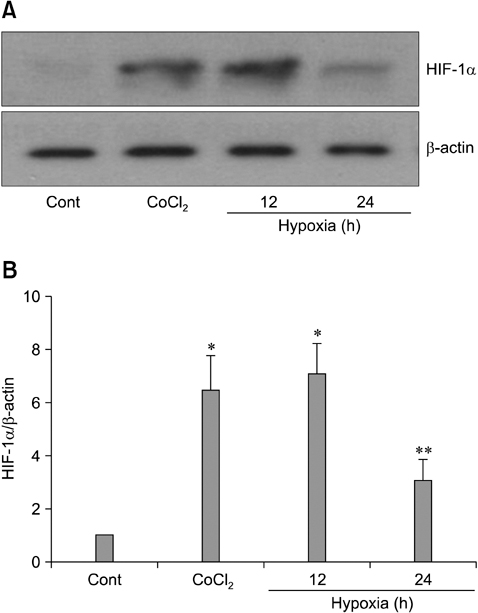
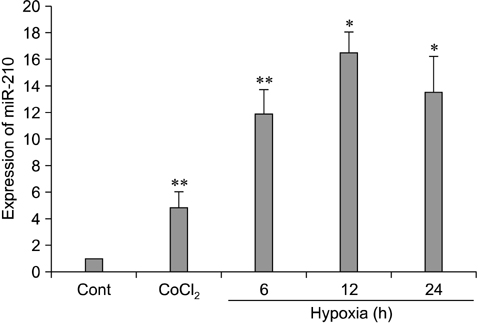
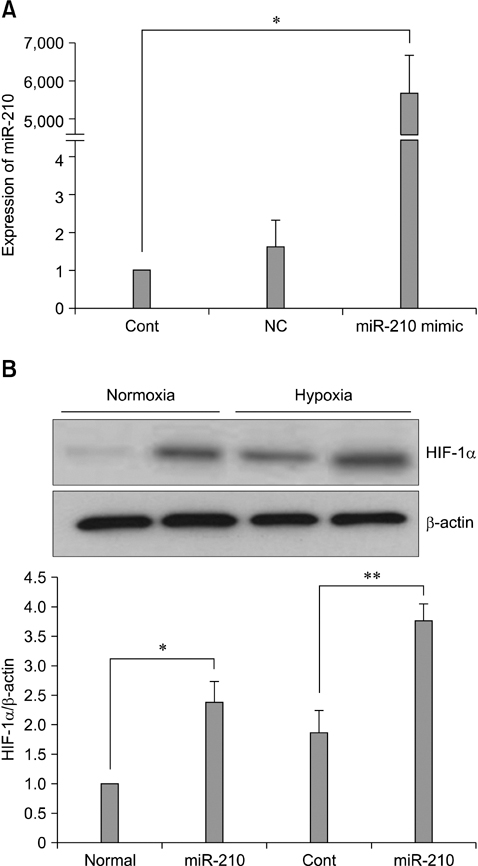
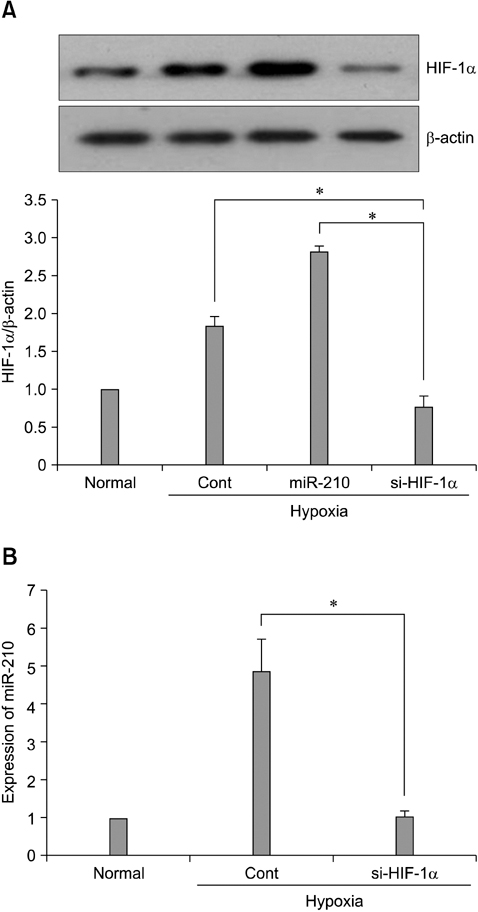
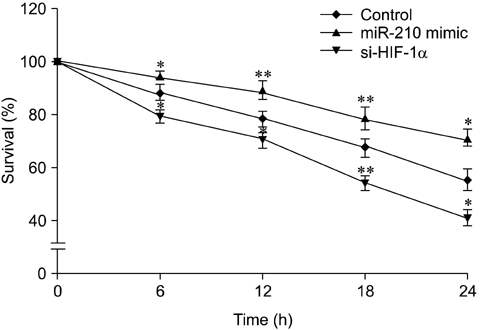
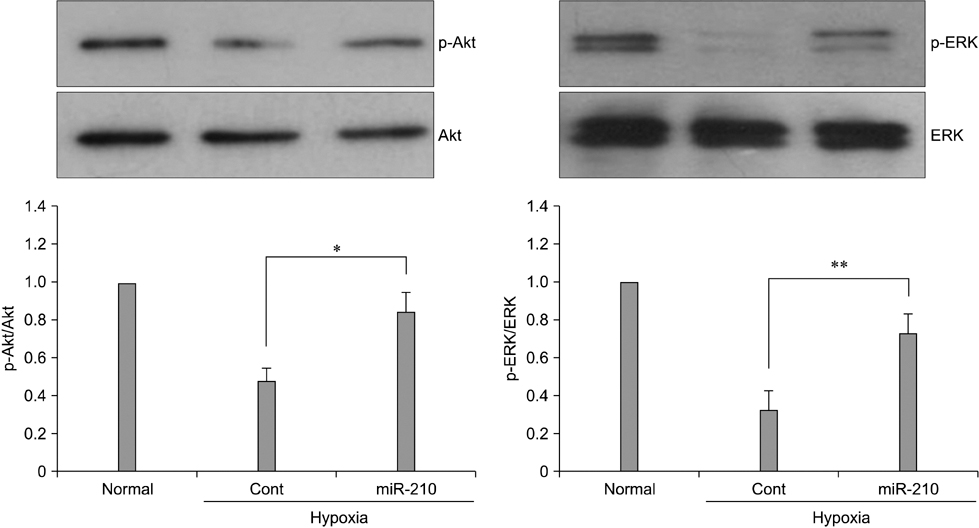
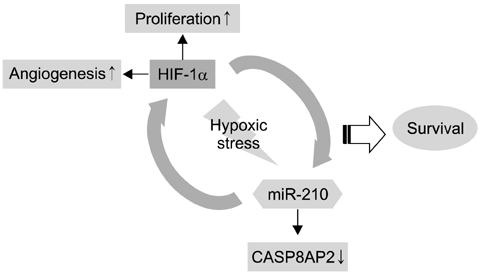
- The use of mesenchymal stem cells (MSCs) has emerged as a potential new treatment for myocardial infarction. However, the poor viability of MSCs after transplantation critically limits the efficacy of this new strategy. The expression of microRNA-210 (miR-210) is induced by hypoxia and is important for cell survival under hypoxic conditions. Hypoxia increases the levels of hypoxia inducible factor-1 (HIF-1) protein and miR-210 in human MSCs (hMSCs). miR-210 positively regulates HIF-1alpha activity. Furthermore, miR-210 expression is also induced by hypoxia through the regulation of HIF-1alpha. To investigate the effect of miR-210 on hMSC survival under hypoxic conditions, survival rates along with signaling related to cell survival were evaluated in hMSCs over-expressing miR-210 or ones that lacked HIF-1alpha expression. Elevated miR-210 expression increased survival rates along with Akt and ERK activity in hMSCs with hypoxia. These data demonstrated that a positive feedback loop involving miR-210 and HIF-1alpha was important for MSC survival under hypoxic conditions.
Keyword
MeSH Terms
-
Cell Survival
Cobalt
Gene Expression Regulation/*physiology
Humans
Hypoxia-Inducible Factor 1, alpha Subunit/genetics/*metabolism
Mesenchymal Stromal Cells/drug effects/metabolism/*physiology
MicroRNAs/*metabolism
Oxygen/pharmacology
*Oxygen Consumption
RNA, Small Interfering/metabolism
Hypoxia-Inducible Factor 1, alpha Subunit
MicroRNAs
RNA, Small Interfering
Cobalt
Oxygen
Figure
Reference
-
1. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004. 116:281–297.2. Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, Ragoussis J. hsa-miR-210 is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res. 2008. 14:1340–1348.
Article3. Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009. 217:318–324.
Article4. Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006. 38:228–233.
Article5. Crosby ME, Kulshreshtha R, Ivan M, Glazer PM. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res. 2009. 69:1221–1229.
Article6. De Filippis L, Delia D. Hypoxia in the regulation of neural stem cells. Cell Mol Life Sci. 2011. 68:2831–2844.
Article7. Dykxhoorn DM. MicroRNAs and metastasis: little RNAs go a long way. Cancer Res. 2010. 70:6401–6406.8. Fasanaro P, D'Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008. 283:15878–15883.
Article9. Felli N, Fontana L, Pelosi E, Botta R, Bonci D, Facchiano F, Liuzzi F, Lulli V, Morsilli O, Santoro S, Valtieri M, Calin GA, Liu CG, Sorrentino A, Croce CM, Peschle C. MicroRNAs 221 and 222 inhibit normal erythropoiesis and erythroleukemic cell growth via kit receptor down-modulation. Proc Natl Acad Sci USA. 2005. 102:18081–18086.
Article10. Geng YJ. Molecular mechanisms for cardiovascular stem cell apoptosis and growth in the hearts with atherosclerotic coronary disease and ischemic heart failure. Ann NY Acad Sci. 2003. 1010:687–697.
Article11. Hu R, Li H, Liu W, Yang L, Tan YF, Luo XH. Targeting miRNAs in osteoblast differentiation and bone formation. Expert Opin Ther Targets. 2010. 14:1109–1120.
Article12. Huang X, Ding L, Bennewith KL, Tong RT, Welford SM, Ang KK, Story M, Le QT, Giaccia AJ. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol Cell. 2009. 35:856–867.
Article13. Huang X, Le QT, Giaccia AJ. MiR-210--micromanager of the hypoxia pathway. Trends Mol Med. 2010. 16:230–237.14. Kelly TJ, Souza AL, Clish CB, Puigserver P. A hypoxia-induced positive feedback loop promotes hypoxia-inducible factor 1α stability through miR-210 suppression of glycerol-3-phosphate dehydrogenase 1-like. Mol Cell Biol. 2011. 31:2696–2706.
Article15. Kim HW, Haider HK, Jiang S, Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009. 284:33161–33168.
Article16. Kloosterman WP, Lagendijk AK, Ketting RF, Moulton JD, Plasterk RHA. Targeted inhibition of miRNA maturation with morpholinos reveals a role for miR-375 in pancreatic islet development. PLoS Biol. 2007. 5:e203.
Article17. Krichevsky AM, Sonntag KC, Isacson O, Kosik KS. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells. 2006. 24:857–864.
Article18. Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, Calin GA, Ivan M. A microRNA signature of hypoxia. Mol Cell Biol. 2007. 27:1859–1867.
Article19. Lei Z, Li B, Yang Z, Fang H, Zhang GM, Feng ZH, Huang B. Regulation of HIF-1α and VEGF by miR-20b tunes tumor cells to adapt to the alteration of oxygen concentration. PLoS One. 2009. 4:e7629.
Article20. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005. 120:15–20.
Article21. Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010. 40:294–309.
Article22. Miranda KC, Huynh T, Tay Y, Ang YS, Tam WL, Thomson AM, Lim B, Rigoutsos I. A pattern-based method for the identification of microRNA binding sites and their corresponding heteroduplexes. Cell. 2006. 126:1203–1217.
Article23. Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, Fujii T, Uematsu M, Ohgushi H, Yamagishi M, Tokudome T, Mori H, Miyatake K, Kitamura S. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005. 112:1128–1135.
Article24. Orbay H, Tobita M, Mizuno H. Mesenchymal stem cells isolated from adipose and other tissues: basic biological properties and clinical applications. Stem Cells Int. 2012. 2012:461718.
Article25. Orlic D, Hill JM, Arai AE. Stem cells for myocardial regeneration. Circ Res. 2002. 91:1092–1102.
Article26. Pedersen IM, Cheng G, Wieland S, Volinia S, Croce CM, Chisari FV, David M. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature. 2007. 449:919–922.
Article27. Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999. 284:1168–1170.
Article28. Puisségur MP, Mazure NM, Bertero T, Pradelli L, Grosso S, Robbe-Sermesant K, Maurin T, Lebrigand K, Cardinaud B, Hofman V, Fourre S, Magnone V, Ricci JE, Pouysségur J, Gounon P, Hofman P, Barbry P, Mari B. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ. 2011. 18:465–478.
Article29. Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J, Vatner DE, Vatner SF, Abdellatif M. Downregulation of miR-199a derepresses hypoxia-inducible factor-1α and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009. 104:879–886.
Article30. Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007. 2007:cm8.
Article31. Silva GV, Litovsky S, Assad JAR, Sousa ALS, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RVC, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005. 111:150–156.
Article32. Taguchi A, Yanagisawa K, Tanaka M, Cao K, Matsuyama Y, Goto H, Takahashi T. Identification of hypoxia-inducible factor-1α as a novel target for miR-17-92 microRNA cluster. Cancer Res. 2008. 68:5540–5545.
Article33. Tay YMS, Tam WL, Ang YS, Gaughwin PM, Yang H, Wang W, Liu R, George J, Ng HH, Perera RJ, Lufkin T, Rigoutsos I, Thomson AM, Lim B. MicroRNA-134 modulates the differentiation of mouse embryonic stem cells, where it causes post-transcriptional attenuation of Nanog and LRH1. Stem Cells. 2008. 26:17–29.
Article34. Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002. 105:93–98.
Article35. Zhang Z, Sun H, Dai H, Walsh RM, Imakura M, Schelter J, Burchard J, Dai X, Chang AN, Diaz RL, Marszalek JR, Bartz SR, Carleton M, Cleary MA, Linsley PS, Grandori C. MicroRNA miR-210 modulates cellular response to hypoxia through the MYC antagonist MNT. Cell Cycle. 2009. 8:2756–2768.
Article36. Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005. 436:214–220.
Article37. Zhu LL, Wu LY, Yew DT, Fan M. Effects of hypoxia on the proliferation and differentiation of NSCs. Mol Neurobiol. 2005. 31:231–242.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Role of HIF1α Regulatory Factors in Stem Cells
- Hypoxic Stress Induces Complement-Mediated Lysis of Mesenchymal Stem Cells by Downregulating Factor H and CD59
- Regulatory Role of Hypoxia Inducible Factor in the Biological Behavior of Nucleus Pulposus Cells
- Improvement of Cell Cycle Lifespan and Genetic Damage Susceptibility of Human Mesenchymal Stem Cells by Hypoxic Priming
- Wheatgrass extract inhibits hypoxia-inducible factor-1-mediated epithelial-mesenchymal transition in A549 cells