Immune Netw.
2009 Aug;9(4):133-137. 10.4110/in.2009.9.4.133.
Further Characterization of Activin A-induced IgA Response in Murine B Lymphocytes
- Affiliations
-
- 1Department of Molecular Bioscience, School of Bioscience and Biotechnology, Kangwon National University, Chuncheon 200-701, Korea. phkim@kangwon.ac.kr
- 2Medical & Bio-material Research Center, Kangwon National University, Chuncheon 200-701, Korea.
- KMID: 1474585
- DOI: http://doi.org/10.4110/in.2009.9.4.133
Abstract
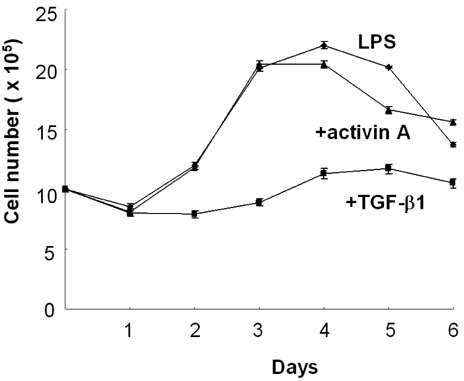
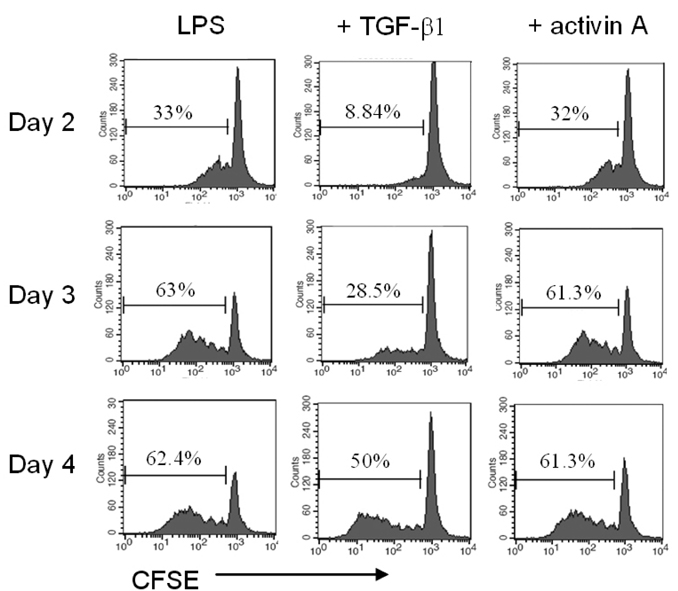
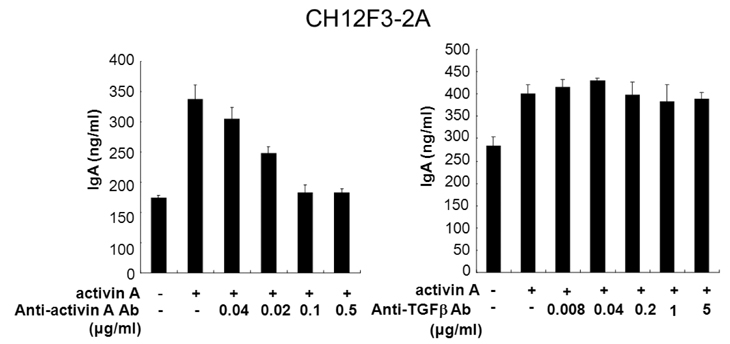
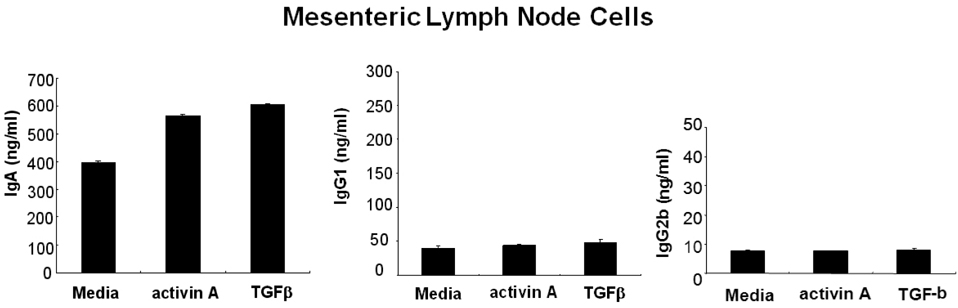
- We have recently shown that activin A, a member of TGF-beta superfamily, stimulates mouse B cells to express IgA isotype but other isotypes. In the present study, we further characterized effects of activin A on B cell growth and IgA expression. We found that activin A did not have effect on LPS-stimulated cell viability. In parallel, CFSE staining analysis revealed that activin A did not alter cell division. An increase of IgA secretion by activin A was completely abrogated by anti-activin A Ab but not by anti-TGFbeta1 Ab. In the same conditions, no other isotypes are significantly affected by each antibody treatment. Finally, activin A, as similar to TGF-beta1, increased IgA secretion by mesenteric lymph node cells. These results suggest that activin A can specifically stimulate IgA response, independent of TGF-beta in the gut.
Keyword
MeSH Terms
Figure
Reference
-
1. Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987. 7:265–276.
Article2. van der Heijden PJ, Stok W, Bianchi AT. Contribution of immunoglobulin-secreting cells in the murine small intestine to the total 'background' immunoglobulin production. Immunology. 1987. 62:551–555.3. Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol. 2003. 3:63–72.
Article4. Kim PH, Kagnoff MF. Transforming growth factor-beta 1 is a costimulator for IgA production. J Immunol. 1990. 144:3411–3416.5. Sonoda E, Matsumoto R, Hitoshi Y, Ishii T, Sugimoto M, Araki S, Tominaga A, Yamaguchi N, Takatsu K. Transforming growth factor beta induces IgA production and acts additively with interleukin 5 for IgA production. J Exp Med. 1989. 170:1415–1420.
Article6. Coffman RL, Lebman DA, Shrader B. Transforming growth factor beta specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J Exp Med. 1989. 170:1039–1044.
Article7. van Vlasselaer P, Punnonen J, de Vries JE. Transforming growth factor-beta directs IgA switching in human B cells. J Immunol. 1992. 148:2062–2067.8. Spieker-Polet H, Yam PC, Arbieva Z, Zhai SK, Knight KL. In vitro induction of the expression of multiple IgA isotype genes in rabbit B cells by TGF-beta and IL-2. J Immunol. 1999. 162:5380–5388.9. McIntyre TM, Klinman DR, Rothman P, Lugo M, Dasch JR, Mond JJ, Snapper CM. Transforming growth factor beta 1 selectivity stimulates immunoglobulin G2b secretion by lipopolysaccharide-activated murine B cells. J Exp Med. 1993. 177:1031–1037.
Article10. Kehrl JH, Taylor A, Kim SJ, Fauci AS. Transforming growth factor-beta is a potent negative regulator of human lymphocytes. Ann N Y Acad Sci. 1991. 628:345–353.
Article11. Smeland EB, Blomhoff HK, Holte H, Ruud E, Beiske K, Funderud S, Godal T, Ohlsson R. Transforming growth factor type beta (TGF beta) inhibits G1 to S transition, but not activation of human B lymphocytes. Exp Cell Res. 1987. 171:213–222.
Article12. Cross D, Cambier JC. Transforming growth factor beta 1 has differential effects on B cell proliferation and activation antigen expression. J Immunol. 1990. 144:432–439.13. Luisi S, Florio P, Reis FM, Petraglia F. Expression and secretion of activin A: possible physiological and clinical implications. Eur J Endocrinol. 2001. 145:225–236.
Article14. Ogawa K, Funaba M, Chen Y, Tsujimoto M. Activin A functions as a Th2 cytokine in the promotion of the alternative activation of macrophages. J Immunol. 2006. 177:6787–6794.
Article15. Lee HJ, Seo GY, Kim HA, Kim PH. Activin A stimulates IgA expression in mouse B cells. Biochem Biophys Res Commun. 2008. 366:574–578.
Article16. Nakamura M, Kondo S, Sugai M, Nazarea M, Imamura S, Honjo T. High frequency class switching of an IgM+ B lymphoma clone CH12F3 to IgA+ cells. Int Immunol. 1996. 8:193–201.
Article17. Park SR, Lee JH, Kim PH. Smad3 and Smad4 mediate transforming growth factor-beta1-induced IgA expression in murine B lymphocytes. Eur J Immunol. 2001. 31:1706–1715.
Article18. Kim PH, Kagnoff MF. Transforming growth factor beta 1 increases IgA isotype switching at the clonal level. J Immunol. 1990. 145:3773–3778.19. Craig SW, Cebra JJ. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971. 134:188–200.
Article20. Santiago AF, Fernandes RM, Santos BP, Assis FA, Oliveira RP, Carvalho CR, Faria AM. Role of mesenteric lymph nodes and aging in secretory IgA production in mice. Cellular immunology. 2008. 253:5–10.
Article21. Kawanishi H, Saltzman L, Strober W. Mechanisms regulating IgA class-specific immunoglobulin production in murine gut-associated lymphoid tissues. II. Terminal differentiation of postswitch sIgA-bearing Peyer's patch B cells. J Exp Med. 1983. 158:649–669.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Immunological abnormalities in patient with IgA nephropathy
- Induction and Regulation of CD30 Expression on Murine B Lymphocytes by Non-specific Stimulation
- Activin A Stimulates Mouse APCs to Express BAFF via ALK4-Smad3 Pathway
- Role of murine Peyer's patch lymphocytes against primary and challenge infections with Cryptosporidium parvum
- Increased mRNA Encoding for Transforming Growth Factor-beta in Peripheral CD4+ Lymphocytes Stimulated with Mitogen from Patients with IgA Nephropathy