Immune Netw.
2011 Aug;11(4):196-202. 10.4110/in.2011.11.4.196.
Activin A Stimulates Mouse APCs to Express BAFF via ALK4-Smad3 Pathway
- Affiliations
-
- 1Department of Molecular Bioscience, College of Biomedical Science, Kangwon National University, Chuncheon 200-701, Korea. phkim@kangwon.ac.kr, gyseo@kangwon.ac.kr
- 2Medical & Bio-material Research Center, Kangwon National University, Chuncheon 200-701, Korea.
- KMID: 1449810
- DOI: http://doi.org/10.4110/in.2011.11.4.196
Abstract
- BACKGROUND
B cell-activating factor belonging to the TNF family (BAFF) is primarily expressed by macrophages and dendritic cells, and stimulates B cell proliferation, differentiation, survival, and Ig production. In the present study, we explored the effect of activin A on BAFF expression by APCs.
METHODS
To investigate the effect of activin A on BAFF expression by mouse APCs, we measured the level of BAFF expression at the transcriptional and protein levels using RT-PCR and ELISA.
RESULTS
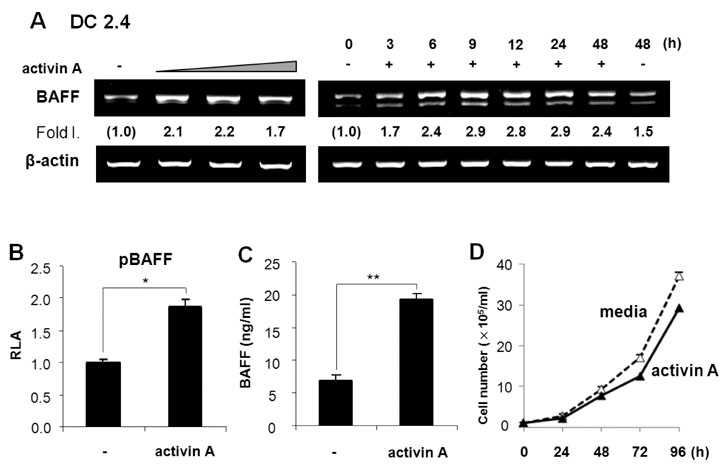
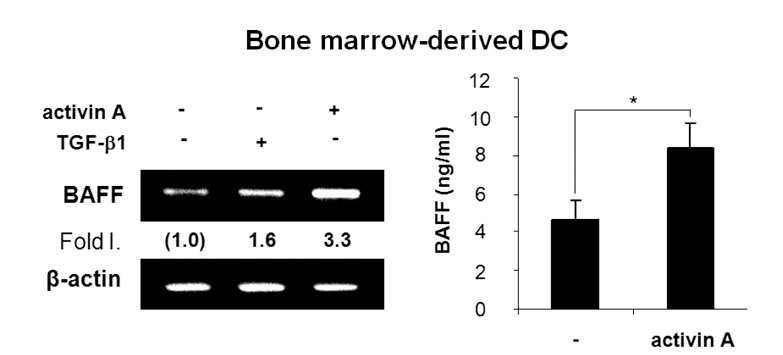
Activin A markedly enhanced BAFF expression in mouse macrophages and dendritic cells at both the transcriptional and protein levels. SB431542, an activin receptor-like kinase 4 (ALK4) inhibitor, completely abrogated activin A-induced BAFF transcription. Furthermore, overexpression of DN-Smad3 abolished activin-induced BAFF expression at the transcriptional and protein levels.
CONCLUSION
These results demonstrate that activin A can enhance BAFF expression through ALK4-Smad3 pathway.
MeSH Terms
Figure
Reference
-
1. Craxton A, Magaletti D, Ryan EJ, Clark EA. Macrophage- and dendritic cell--dependent regulation of human B-cell proliferation requires the TNF family ligand BAFF. Blood. 2003. 101:4464–4471.
Article2. Dubois B, Massacrier C, Vanbervliet B, Fayette J, Brière F, Banchereau J, Caux C. Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J Immunol. 1998. 161:2223–2231.3. Dubois B, Vanbervliet B, Fayette J, Massacrier C, Van Kooten C, Brière F, Banchereau J, Caux C. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J Exp Med. 1997. 185:941–951.
Article4. Fagarasan S, Honjo T. T-Independent immune response: new aspects of B cell biology. Science. 2000. 290:89–92.
Article5. Snapper CM, Mond JJ. A model for induction of T cell-independent humoral immunity in response to polysaccharide antigens. J Immunol. 1996. 157:2229–2233.6. Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, Tschopp J, Cachero TG, Batten M, Wheway J, Mauri D, Cavill D, Gordon TP, Mackay CR, Mackay F. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren's syndrome. J Clin Invest. 2002. 109:59–68.
Article7. Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, Valmori D, Romero P, Werner-Favre C, Zubler RH, Browning JL, Tschopp J. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999. 189:1747–1756.
Article8. Mackay F, Ambrose C. The TNF family members BAFF and APRIL: the growing complexity. Cytokine Growth Factor Rev. 2003. 14:311–324.
Article9. Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002. 2:465–475.
Article10. Sutherland AP, Mackay F, Mackay CR. Targeting BAFF: immunomodulation for autoimmune diseases and lymphomas. Pharmacol Ther. 2006. 112:774–786.
Article11. Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000. 102:553–563.
Article12. Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002. 3:822–829.
Article13. Kim PH, Kagnoff MF. Transforming growth factor beta 1 increases IgA isotype switching at the clonal level. J Immunol. 1990. 145:3773–3778.14. Lebman DA, Lee FD, Coffman RL. Mechanism for transforming growth factor beta and IL-2 enhancement of IgA expression in lipopolysaccharide-stimulated B cell cultures. J Immunol. 1990. 144:952–959.15. McIntyre TM, Klinman DR, Rothman P, Lugo M, Dasch JR, Mond JJ, Snapper CM. Transforming growth factor beta 1 selectivity stimulates immunoglobulin G2b secretion by lipopolysaccharide-activated murine B cells. J Exp Med. 1993. 177:1031–1037.
Article16. Kim HA, Jeon SH, Seo GY, Park JB, Kim PH. TGF-beta1 and IFN-gamma stimulate mouse macrophages to express BAFF via different signaling pathways. J Leukoc Biol. 2008. 83:1431–1439.
Article17. Massagué J. TGF-beta signal transduction. Annu Rev Biochem. 1998. 67:753–791.18. Lebrun JJ, Chen Y, Vale W. Aono T, Sugino H, Vale WW, editors. Receptor serine kinases and signaling by activins and inhibins. Inhibin, Activins and Flollistatin: regulatory functions in system and cell biology. 1997. New York: Springer-Verlag New York;1–20.
Article19. Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003. 113:685–700.
Article20. Lee HJ, Seo GY, Kim HA, Kim PH. Activin A stimulates IgA expression in mouse B cells. Biochem Biophys Res Commun. 2008. 366:574–578.
Article21. Park SR, Lee JH, Kim PH. Smad3 and Smad4 mediate transforming growth factor-beta1-induced IgA expression in murine B lymphocytes. Eur J Immunol. 2001. 31:1706–1715.
Article22. Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, Soppet D, Charters M, Gentz R, Parmelee D, Li Y, Galperina O, Giri J, Roschke V, Nardelli B, Carrell J, Sosnovtseva S, Greenfield W, Ruben SM, Olsen HS, Fikes J, Hilbert DM. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999. 285:260–263.
Article23. Nardelli B, Belvedere O, Roschke V, Moore PA, Olsen HS, Migone TS, Sosnovtseva S, Carrell JA, Feng P, Giri JG, Hilbert DM. Synthesis and release of B-lymphocyte stimulator from myeloid cells. Blood. 2001. 97:198–204.
Article24. Chen YG, Wang Q, Lin SL, Chang CD, Chuang J, Ying SY. Activin signaling and its role in regulation of cell proliferation, apoptosis, and carcinogenesis. Exp Biol Med (Maywood). 2006. 231:534–544.
Article25. Huang HM, Chang TW, Liu JC. Basic fibroblast growth factor antagonizes activin A-mediated growth inhibition and hemoglobin synthesis in K562 cells by activating ERK1/2 and deactivating p38 MAP kinase. Biochem Biophys Res Commun. 2004. 320:1247–1252.
Article26. Ogihara T, Watada H, Kanno R, Ikeda F, Nomiyama T, Tanaka Y, Nakao A, German MS, Kojima I, Kawamori R. p38 MAPK is involved in activin A- and hepatocyte growth factor-mediated expression of pro-endocrine gene neurogenin 3 in AR42J-B13 cells. J Biol Chem. 2003. 278:21693–21700.
Article27. Zhang L, Deng M, Parthasarathy R, Wang L, Mongan M, Molkentin JD, Zheng Y, Xia Y. MEKK1 transduces activin signals in keratinocytes to induce actin stress fiber formation and migration. Mol Cell Biol. 2005. 25:60–65.
Article28. Castigli E, Wilson SA, Scott S, Dedeoglu F, Xu S, Lam KP, Bram RJ, Jabara H, Geha RS. TACI and BAFF-R mediate isotype switching in B cells. J Exp Med. 2005. 201:35–39.
Article29. Yamada T, Zhang K, Yamada A, Zhu D, Saxon A. B lymphocyte stimulator activates p38 mitogen-activated protein kinase in human Ig class switch recombination. Am J Respir Cell Mol Biol. 2005. 32:388–394.
Article30. Kim HA, Seo GY, Kim PH. Macrophage-derived BAFF induces AID expression through the p38MAPK/CREB and JNK/AP-1 pathways. J Leukoc Biol. 2011. 89:393–398.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lactoferrin Stimulates Mouse Macrophage to Express BAFF via Smad3 Pathway
- Further Characterization of Activin A-induced IgA Response in Murine B Lymphocytes
- A high concentration of genistein down-regulates activin A, Smad3 and other TGF-beta pathway genes in human uterine leiomyoma cells
- TGF-beta1 induces mouse dendritic cells to express VEGF and its receptor (Flt-1) under hypoxic conditions
- B cell activation factor (BAFF) is a novel adipokine that links obesity and inflammation