Immune Netw.
2009 Aug;9(4):122-126. 10.4110/in.2009.9.4.122.
Regulation of Tumor Immune Surveillance and Tumor Immune Subversion by TGF-beta
- Affiliations
-
- 1Laboratory of Immunology, Lee Gil-Ya Cancer and Diabetes Institute, Gachon University of Medicine and Science, Incheon, Korea. mamurashin@gachon.ac.kr
- 2Laboratory of Cancer Biology and Genetics, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
- KMID: 1474583
- DOI: http://doi.org/10.4110/in.2009.9.4.122
Abstract
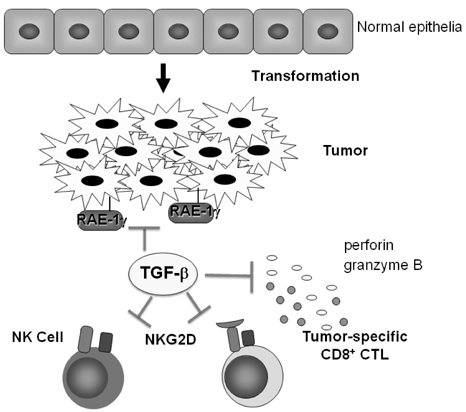
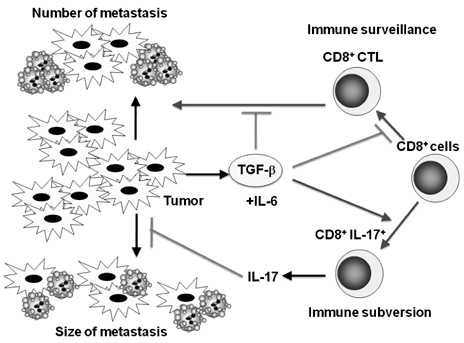
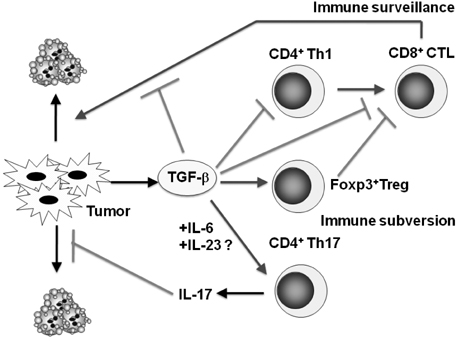
- Transforming growth factor-beta (TGF-beta) is a highly pleiotropic cytokine playing pivotal roles in immune regulation. TGF-beta facilitates tumor cell survival and metastasis by targeting multiple cellular components. Focusing on its immunosuppressive functions, TGF-beta antagonists have been employed for cancer treatment to enhance tumor immunity. TGF-beta antagonists exert anti-tumor effects through #1 activating effector cells such as NK cells and cytotoxic CD8+ T cells (CTLs), #2 inhibiting regulatory/suppressor cell populations, #3 making tumor cells visible to immune cells, #4 inhibiting the production of tumor growth factors. This review focuses on the effect of TGF-beta on T cells, which are differentiated into effector T cells or newly identified tumor-supporting T cells.
MeSH Terms
Figure
Reference
-
1. Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol. 2001. 187:265–276.2. Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006. 24:99–146.3. Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A. 2003. 100:8621–8623.4. Yang YA, Dukhanina O, Tang B, Mamura M, Letterio JJ, MacGregor J, Patel SC, Khozin S, Liu ZY, Green J, Anver MR, Merlino G, Wakefield LM. Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J Clin Invest. 2002. 109:1607–1615.
Article5. Ge R, Rajeev V, Ray P, Lattime E, Rittling S, Medicherla S, Protter A, Murphy A, Chakravarty J, Dugar S, Schreiner G, Barnard N, Reiss M. Inhibition of growth and metastasis of mouse mammary carcinoma by selective inhibitor of transforming growth factor-beta type I receptor kinase in vivo. Clin Cancer Res. 2006. 12:4315–4330.
Article6. Wrzesinski SH, Wan YY, Flavell RA. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin Cancer Res. 2007. 13:5262–5270.
Article7. Denoix PF. Enquete permanent dans les centres anticancereaux. Bull Inst Nat Hyg. 1946. 1:70–75.8. Chen F, Fujinaga T, Sato K, Sonobe M, Shoji T, Sakai H, Miyahara R, Bando T, Okubo K, Hirata T, Toi M, Date H. Clinical features of surgical resection for pulmonary metastasis from breast cancer. Eur J Surg Oncol. 2009. 35:393–397.
Article9. Humphrey LJ, Singla O, Volenec FJ. Immunologic responsiveness of the breast cancer patient. Cancer. 1980. 46:893–898.
Article10. Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005. 202:919–929.
Article11. Alleva DG, Burger CJ, Elgert KD. Tumor-induced regulation of suppressor macrophage nitric oxide and TNF-alpha production. Role of tumor-derived IL-10, TGF-beta, and prostaglandin E2. J Immunol. 1994. 153:1674–1686.12. Bierie B, Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer. 2006. 6:506–520.13. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008. 454:436–444.
Article14. Yang L, Moses HL. Transforming growth factor beta: tumor suppressor or promoter? Are host immune cells the answer? Cancer Res. 2008. 68:9107–9111.
Article15. Geiser AG, Letterio JJ, Kulkarni AB, Karlsson S, Roberts AB, Sporn MB. Transforming growth factor beta 1 (TGF-beta 1) controls expression of major histocompatibility genes in the postnatal mouse: aberrant histocompatibility antigen expression in the pathogenesis of the TGF-beta 1 null mouse phenotype. Proc Natl Acad Sci U S A. 1993. 90:9944–9948.
Article16. Ljunggren HG, Kärre K. In search of the 'missing self: MHC molecules and NK cell recognition. Immunol Today. 1990. 11:237–244.
Article17. Zwirner NW, Fuertes MB, Girart MV, Domaica CI, Rossi LE. Cytokine-driven regulation of NK cell functions in tumor immunity: role of the MICA-NKG2D system. Cytokine Growth Factor Rev. 2007. 18:159–170.
Article18. Lee JC, Lee KM, Kim DW, Heo DS. Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol. 2004. 172:7335–7340.
Article19. Nam JS, Terabe M, Mamura M, Kang MJ, Chae H, Stuelten C, Kohn E, Tang B, Sabzevari H, Anver MR, Lawrence S, Danielpour D, Lonning S, Berzofsky JA, Wakefield LM. An anti-transforming growth factor beta antibody suppresses metastasis via cooperative effects on multiple cell compartments. Cancer Res. 2008. 68:3835–3843.
Article20. Thomas DA, Massagué J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005. 8:369–380.
Article21. Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Semin Cancer Biol. 2006. 16:115–123.
Article22. Kitamura T, Kometani K, Hashida H, Matsunaga A, Miyoshi H, Hosogi H, Aoki M, Oshima M, Hattori M, Takabayashi A, Minato N, Taketo MM. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat Genet. 2007. 39:467–475.
Article23. Nam JS, Terabe M, Kang MJ, Chae H, Voong N, Yang YA, Laurence A, Michalowska A, Mamura M, Lonning S, Berzofsky JA, Wakefield LM. Transforming growth factor beta subverts the immune system into directly promoting tumor growth through interleukin-17. Cancer Res. 2008. 68:3915–3923.
Article24. Kapp JA, Bucy RP. CD8+ suppressor T cells resurrected. Hum Immunol. 2008. 69:715–720.
Article25. Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002. 17:375–387.
Article26. Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGF beta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006. 24:179–189.
Article27. Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17- producing T cells in ovarian cancer. Proc Natl Acad Sci U S A. 2008. 105:15505–15510.
Article28. Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003. 101:2620–2627.
Article29. Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009. 114:357–359.
Article30. Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009. 206:1457–1464.
Article31. McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009. 10:314–324.
Article32. Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, Yu H. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009. 15:114–123.
Article33. Martin-Orozco N, Dong C. The IL-17/IL-23 axis of inflammation in cancer: friend or foe? Curr Opin Investig Drugs. 2009. 10:543–549.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Advances in the Development of TGF-β Signaling Inhibitors for Anticancer Therapy
- The effect of transforming growth factor-beta on the expression of CD8 in the CTLL-2 cell line
- Insights into the Transforming Growth Factor-beta Signaling Pathway in Cutaneous Melanoma
- Immunohistochemical Study of TGF- type I and type II receptor Expression in Psoriatic Epidermis
- Stimulatory Effect of IL-10 on Antitumor Cytolytic Activity of Murine Spleen Cells