J Bacteriol Virol.
2009 Mar;39(1):29-39. 10.4167/jbv.2009.39.1.29.
Identification of Shiga Toxin-producing E. coli Isolated from Diarrhea Patients and Cattle in Gwangju Area, Korea
- Affiliations
-
- 1Health & Environment Institute of Gwangju, Gwangju, Korea. vetmj@korea.kr
- 2Department of Veterinary Medicine, Chonnam National University, Gwangju, Korea.
- KMID: 1474178
- DOI: http://doi.org/10.4167/jbv.2009.39.1.29
Abstract
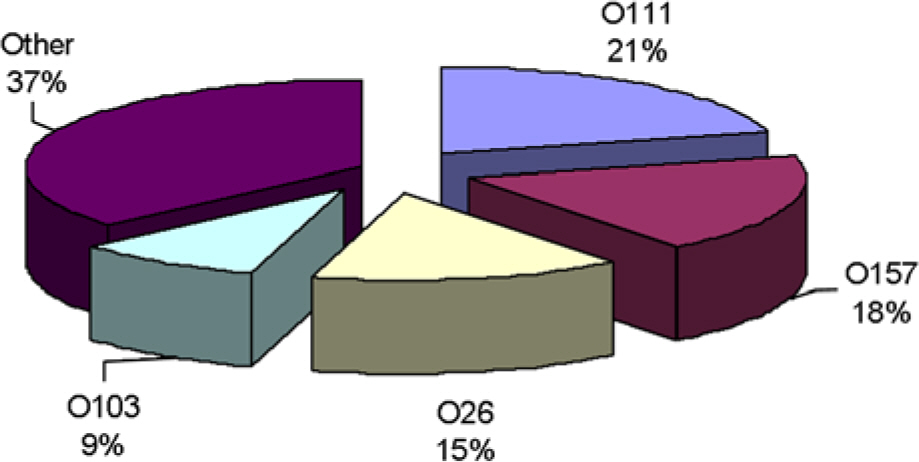
- Shiga toxin-producing Escherichia coli (STEC) strains are commensal bacteria in cattle and cause food borne disease in human. We analyzed the isolation rate of STEC in stool specimens of patients with diarrhea and in fecal samples of cattle in Gwangju, Korea. STEC strains were detected from 33 (0.19%) out of 17,148 patients with diarrhea while there has been a progressive increase in the incidence rate from 0.07% in 2004 to 0.33% in 2008. We investigated serotypes, shiga toxin genes, and antimicrobial resistance patterns of the 44 STEC isolates from human and cattle sources. The 33 STEC isolates from human belonged to 14 O serotypes including O157, O26 and O111. The 11 isolates from cattle belonged to 11 O serotypes. PCR detection for stx genes showed that 12 (27.3%) isolates carried stx1 genes, 20 (45.5%) possessed stx2 genes, and 12 (27.3%) carried both stx1 and stx2. Of the 33 STEC isolates from human, 25 strains (76%) were resistant to one or more antibiotics. High level of resistance to tetracycline (73%) was most common, followed by ticarcillin and ampicillin (64%). But none of the 33 isolates from human were resistant to amikacin, cefazolin, cefepime, cefotetan, cefotaxime, ciprofloxacin, or imipenem. The 5 strains (45%) of the 11 isolates from cattle were resistant to at least one or three antibiotics but most of the isolates were sensitive to the 16 antibiotics employed in this survey. In conclusion, toxin types and serotypes of STEC isolated from human and cattle were diverse, and non-O157 STEC was also observed to be a greater proportion of STEC isolates. According to a specific comparison solely based on the toxin types and serotypes, most of the STEC strains isolated from cattle feces in Gwangju, Korea showed characteristics different from those isolated from patients. Therefore, laboratory surveillance is required to detect and carefully monitor the potentially hypervirulent STEC not only in human and cattle but also in other animals.
MeSH Terms
-
Amikacin
Ampicillin
Animals
Anti-Bacterial Agents
Bacteria
Cattle
Cefazolin
Cefotaxime
Cefotetan
Cephalosporins
Ciprofloxacin
Diarrhea
Feces
Humans
Imipenem
Incidence
Korea
Organothiophosphorus Compounds
Polymerase Chain Reaction
Shiga Toxin
Shiga-Toxigenic Escherichia coli
Tetracycline
Ticarcillin
Amikacin
Ampicillin
Anti-Bacterial Agents
Cefazolin
Cefotaxime
Cefotetan
Cephalosporins
Ciprofloxacin
Imipenem
Organothiophosphorus Compounds
Shiga Toxin
Tetracycline
Ticarcillin
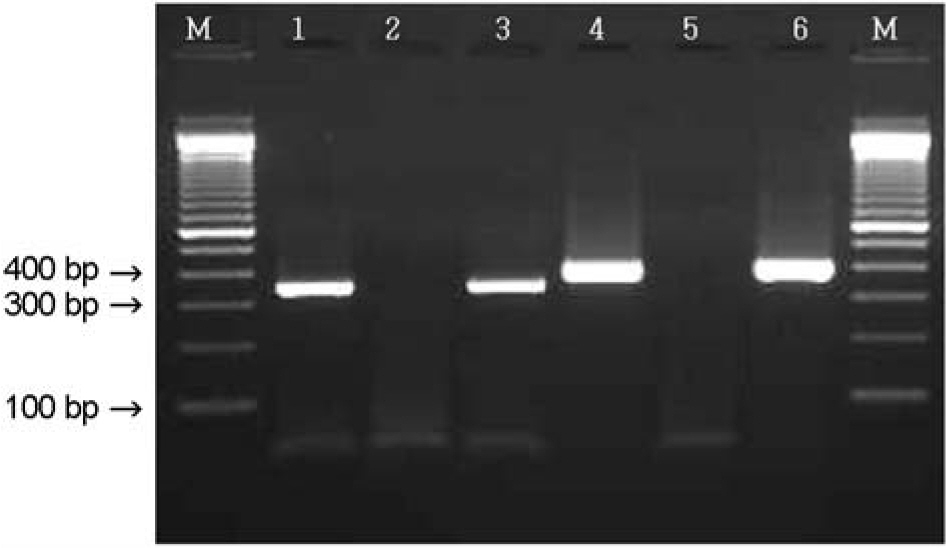
Figure
Cited by 2 articles
-
Guideline for the Antibiotic Use in Acute Gastroenteritis
Youn Jeong Kim, Ki-Ho Park, Dong-Ah Park, Joonhong Park, Byoung Wook Bang, Seung Soon Lee, Eun Jung Lee, Hyo-Jin Lee, Sung Kwan Hong, Yang Ree Kim
Infect Chemother. 2019;51(2):217-243. doi: 10.3947/ic.2019.51.2.217.Food poisoning
Chang-Beom Ryu, Moon-Sung Lee
J Korean Med Assoc. 2011;54(6):617-626. doi: 10.5124/jkma.2011.54.6.617.
Reference
-
1). Griffin PM. Epidemiology of Shiga toxin-producing Escherichia coli infections in humans in the United States. Kaper J. B., O'Brien A. D., editors(eds.). Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. ASM Press;Washington D.C.: 1998. pp.p. 15–22.2). Jaeger JL., Acheson DW. Shiga toxin-producing Escherichia coli. Curr Infect Dis Rep. 2000. 2:61–7.3). Wade WG., Thom BT., Evans N. Cytotoxic enteropathogenic Escherichia coli. Lancet. 1979. 2:1235–6.4). 국립예방위생연구소. 장관출혈성대장균 O157:H7? 집 단발생. 병원미생물검출정보. 1996. 17:180–90.5). Bell C. Approach to the control of entero-haemorrhagic Escherichia coli (EHEC). Int J Food Microbiol. 2002. 78:197–216.6). Bettelheim KA., Beutin L. Rapid laboratory identification and characterization of verocytotoxigenic (Shiga toxin producing) Escherichia coli (VETC/STEC). J Appl Microbiol. 2003. 95:205–17.7). Konowalchuk J., Speirs JL., Stavric S. Vero response to a cytotoxin of Escherichia coli. Infect Immun. 1977. 18:775–9.8). Scheutz F., Beutin L., Pierard D., Smith HR. Appendix: Nomenclature of verocytotoxins. Duffy G, Garvey P, McDowell D, editors. (eds). Verocytotoxigenic E. coli. Food and Nutrition Press, Inc.;Connecticut: 2001.9). Ziebell KA., Read SC., Johnson RP., Gyles CL. Evaluation of PCR and PCR-RFLP protocols for identifying Shiga toxins. Res Microbiol. 2002. 153:289–300.
Article10). WHO. Zoonotic non-O157 Shiga toxin-producing Escherichia coli (STEC): report of a W.H.O. Scientific Working Group Meeting, Berlin, Germany, 22 to 23 June 1998. World Health Organization, Geneva, Switzerland. 1999. ;p.1–30.11). Bertin Y., Boukhors K., Livrelli V., Martin C. Localization of the insertion site and pathotype determination of the locus of enterocyte effacement of Shiga toxin-producing Escherichia coli strains. Appl Environ Microbiol. 2004. 70:61–8.12). Scheutz F., Cheasty T., Woodward D., Smith HR. Designation of O174 and O175 to temporary O groups OX3 and OX7, and six new E. coli O groups that include Verocytotoxin-producing E. coli (VTEC): O176, O177, O178, O179, O180 and O181. APMIS. 2004. 112:569–84.13). Paton AW., Paton JC. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J Clin Microbiol. 1998. 36:598–602.14). Bertin Y., Boukhors K., Pradel N., Livrelli V., Martin C. Stx2 subtyping of Shiga toxin-producing Escherichia coli isolated from cattle in France: detection of a new Stx2 subtype and correlation with additional virulence factors. J Clin Microbiol. 2001. 39:3060–5.15). Bürk C., Dietrich R., Acar G., Moravek M., Bülte M., Märtlbauer E. Identification and characterization of a new variant of Shiga toxin 1 in Escherichia coli ONT:H19 of bovine origin. J Clin Microbiol. 2003. 41:2106–12.16). Johnson WM., Pollard DR., Lior H., Tyler SD., Rozee KR. Differentiation of genes coding for Escherichia coli verotoxin 2 and the verotoxin associated with porcine edema disease (VTe) by the polymerase chain reaction. J Clin Microbiol. 1990. 28:2351–3.17). Pie'rard D., Muyldermans G., Moriau L., Stevens D., Lauwers S. Identification of new verocytotoxin type 2 variant B-subunit genes in human and animal Escherichia coli isolates. J Clin Microbiol. 1998. 36:3317–22.18). Unkmeir A., Schmidt H. Structural analysis of phage-borne stx genes and their flanking sequences in Shiga toxin-producing Escherichia coli and Shigella dysenteriae type 1 strains. Infect Immun. 2000. 68:4856–64.19). Friedrich AW., Bielaszewska M., Zhang WL., Pulz M., Kuczius T., Ammon A., Karch H. Escherichia coli harboring Shiga toxin 2 gene variants: Frequency and association with clinical symptoms. J Infect Dis. 2002. 185:74–84.20). Ramachandran V., Hornitzky MA., Bettelheim KA., Walker MJ., Djordjevic SP. The common ovine Shiga toxin 2-containing Escherichia coli serotypes and human isolates of the same serotypes possess a Stx2d toxin type. J Clin Microbiol. 2001. 39:1932–7.21). Pass MA., Odedra R., Batt RM. Multiplex PCRs for identification of Escherichia coli virulence genes. J Clin Microbiol. 2000. 38:2001–4.22). Suzuki M., Kondo F., Ito Y., Matsumoto M., Hata M., Oka H., Takahashi M., Sakae K. Identification of a Shiga-toxin type I variant containing an IS1203-like element, from Shiga-toxin producing Escherichia coli. FEMS Microbiol Lett. 2004. 234:63–7.23). Pan TM., Chen LM., Su YC. Identification of Escherichia coil O157:H7 by multiplex PCR with primer specific to the hlyA, eaeA, stx1, stx2, fliC, and rfb genes. J Formos Med Assoc. 2002. 101:661–4.24). Deibel C., Kramer S., Chakraborty T., Ebel F. EspE, a novel secreted protein of attaching and effacing bacteria, is directly translocated into infected host cells, where it appears as a tyrosine-phosphorylated 90 kDa protein. Mol Microbiol. 1998. 28:463–74.25). Aranda KR., Fagundes-Neto U., Scaletsky IC. Evaluation of multiplex PCRs for diagnosis of infection with diarrheagenic Escherichia coil and Shigella spp. J Clin Microbiol. 2004. 42:5849–53.26). Yamamoto T., Echeverria P. Detection of the enteroaggregative Escherichia coil heat-stable enterotoxin 1 gene sequences in enterotoxigenic E. coil strains pathogenic for humans. Infect Immun. 1996. 64:1441–5.27). Wood PK., Morris JG Jr., Small PL., Sethabutr O., Toledo MR., Trabulsi L., Kaper JB. Comparison of DNA probes and the sereny test for identification of invasive Shigella and Escherichia coli strains. J Clin Microbiol. 1986. 24:498–500.28). NCCLS. Performance standards for antimicrobiol disk susceptibility test. 10th ed. 2008. 29:M02–A10.29). Pradel N., Livrelli V., De Champs C., Palcoux JB., Reynaud A., Scheutz F., Sirot J., Joly B., Forestier C. Prevalence and characterization of Shiga toxin-producing Escherichia coli isolated from cattle, food, and children during a one-year prospective study in France. J Clin Microbiol. 2000. 38:1023–31.30). Welinder-Olsson C., Stenqvist K., Badenfors M., Branberg A., Floren K., Holm M., Holmberg L., Kjellin E., Marild S., Studahl A., Kaijser B. EHEC outbreak among staff at a children's hospital-use of PCR for verocytotoxin detection and PFGE for epidemiological investigatin. Epidemiol Infect. 2004. 132:43–9.31). 오명돈, 최강원. 감염질환. 도서출한 한의학, 제1판. 2000. p.195–8.32). Zweifel C., Blanco JE., Blanco M., Balnco J., Stephan R. Serotypes and virulence genes of ovine non-O157 shiga toxin-producing Escherichia coli in Switzerland. Int J Food Microbiol. 2004. 95:19–27.33). Nataro JP., Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998. 11:142–201.34). Wilson JB., McEwen SA., Clarke RC., Leslie KE., Waltner-Toews D., Gyles CL. A case-control study of selected pathogens including verocytotoxigenic Escherichia coli in calf diarrhea on an Ontario veal farm. Can J Vet Res. 1992. 56:184–8.35). Cerqueira AM., Guth BE., Joaquim RM., Andrade JR. High occurrence of shiga toxin-producing Escherichia coli (STEC) in healthy cattle in Rio de Janeiro State, Brazil. Vet Microbiol. 1999. 70:111–21.36). Thran BH., Hussein HS., Hall MR., Khaiboullina SF. Shiga toxin-producing Escherichia coli in beef heifers grazing an irrigated pasture. J Food Prot. 2001. 64:1613–6.37). Lee H-S. Serotype distribution and genetic characteristics of VTEC in Korean animal. 104th ASM general meeting. poster. 2004.38). Blanco M., Blanco JE., Mora A., Dahbi G., Alonso MP., Gonzalez EA., Bernardez MI., Blanco J. Serotypes, virulence genes, and intimin types of Shiga toxin (verotoxin)-producing Escherichia coli isolates from cattle in Spain and identification of a new intimin variant gene (eae-xi). J Clin Microbiol. 2004. 42:645–51.39). Prager R., Annemuller S., Tschape H. Diversity of virulence patterns among shiga toxin-producing Escherichia coli from human clinical cases-need for more detailed diagnostics. Int J Med Microbiol. 2005. 295:29–38.40). Karmali MA., Mascarenhas M., Shen S., Ziebell K., Johnson S., Reid-Smith R., Isaac-Renton J., Clark C., Rahn K., Kaper JB. Association of genomic O island 122 of Escherichia coli EDL 933 with verocytotoxin-producing Escherichia coli seropatho-types that are linked to epidemic and/or serious disease. J Clin Microbiol. 2003. 41:4930–40.41). Nielsen EM., Scheutz F., Torpdahl M. Continuous surveillance of Shiga toxin-producing Escherichia coli infections by pulsed-field gel electrophoresis shows that most infections are sporadic. Foodborne Pathog Dis. 2006. 3:81–7.42). Jelacic JK., Damrow T., Chen GS., Jelacic S., Bielaszewska M., Ciol M., Carvalho HM., Melton-Celsa AR., O'Brien AD., Tarr PI. Shiga toxin-producing Escherichia coli in Montana: bacterial genotypes and clinical profiles. J Infect Dis. 2003. 188:719–29.43). Brooks JT., Sowers EG., Wells JG., Greene KD., Griffin PM., Hoekstra RM., Strockbine NA. Non-O157 Shiga toxin-producing Escherichia coli infections in the United States, 1983-2002. J Infect Dis. 2005. 192:1422–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epidemiological Analysis of Shiga Toxin-producing E. coli Isolated in Gwangju, Korea, by Pulse-field Gel Electrophoresis
- Molecular Genetic Characterization of Shiga Toxin-producing E. coli Isolated from Diarrhea Patients and Cattle in Gwangju Area, Korea
- Development of a multiplex loop-mediated isothermal amplification assay to detect shiga toxin-producing Escherichia coli in cattle
- Isolation of Escherichia coli O157 in Children with Diarrhea
- Virulence factors in Escherichia coli isolated from calves with diarrhea in Vietnam