J Bacteriol Virol.
2009 Sep;39(3):217-227. 10.4167/jbv.2009.39.3.217.
In vitro Expansion of Natural Regulatory T Lymphocytes Useful for Cell Therapy in Allotransplantation
- Affiliations
-
- 1Department of Surgery, Ulsan University College of Medicine & Asan Medical Center, Asan Institute for Life Sciences, Seoul, Korea. dglim@amc.seoul.kr
- KMID: 1474164
- DOI: http://doi.org/10.4167/jbv.2009.39.3.217
Abstract
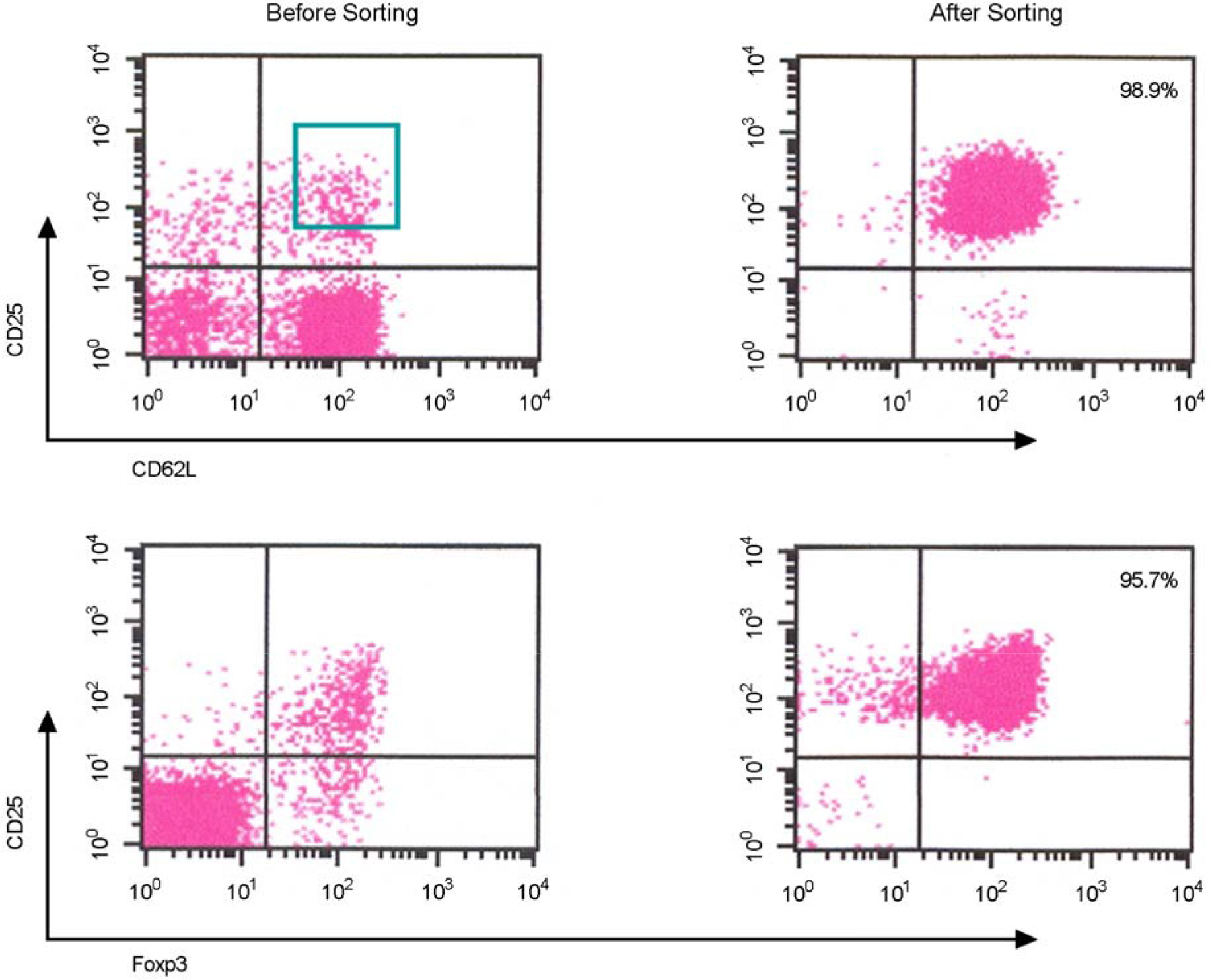
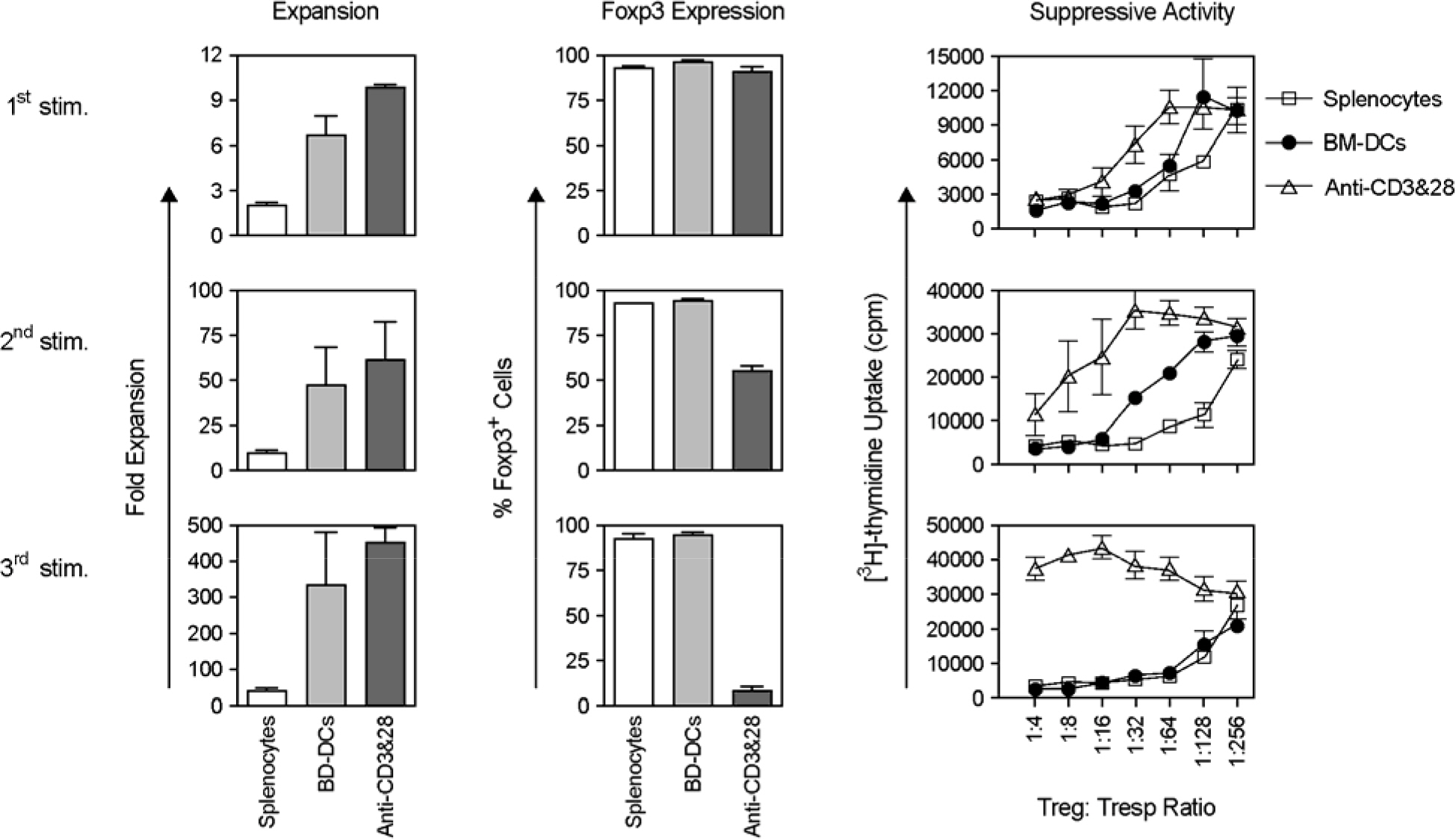
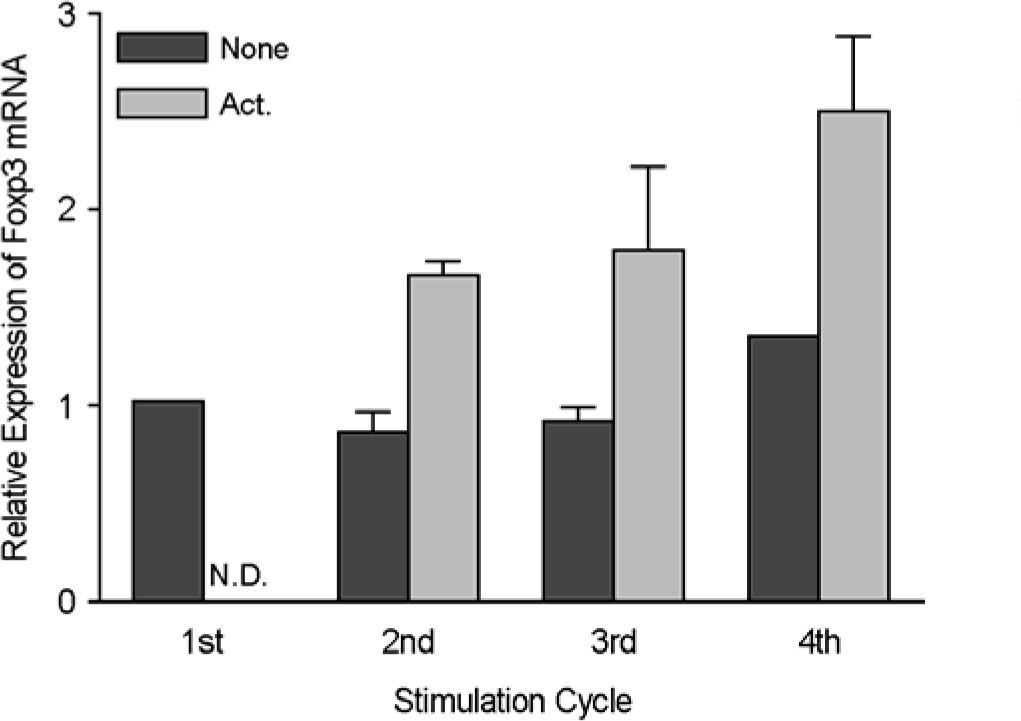
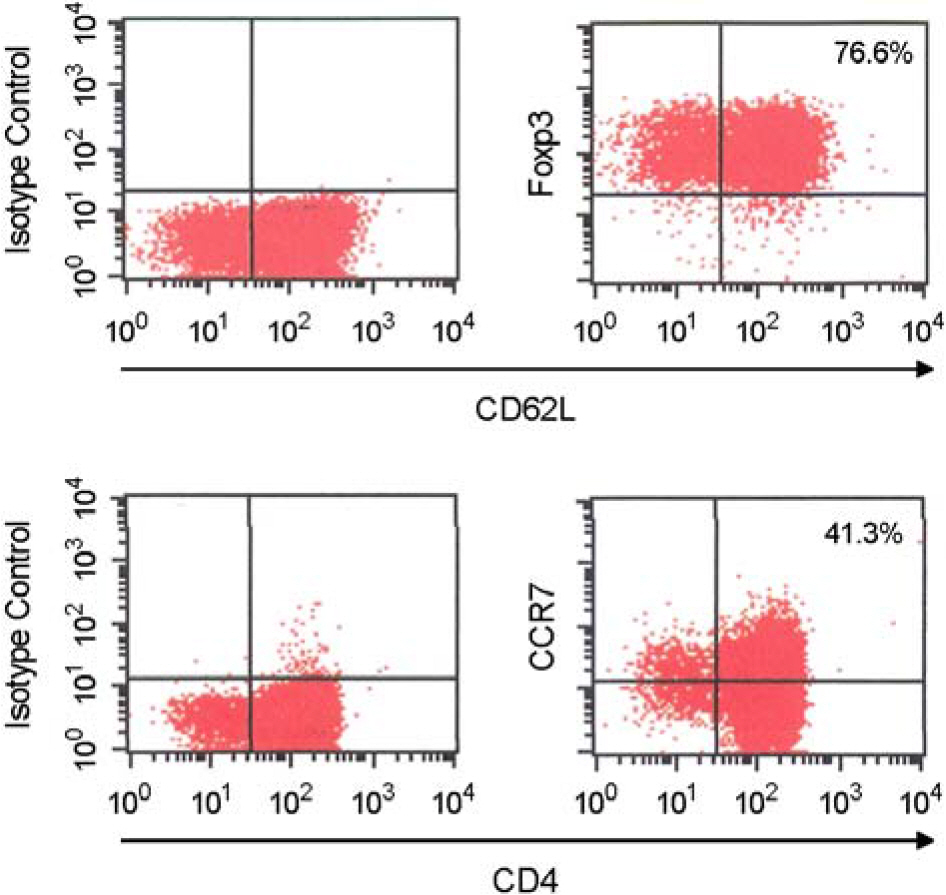
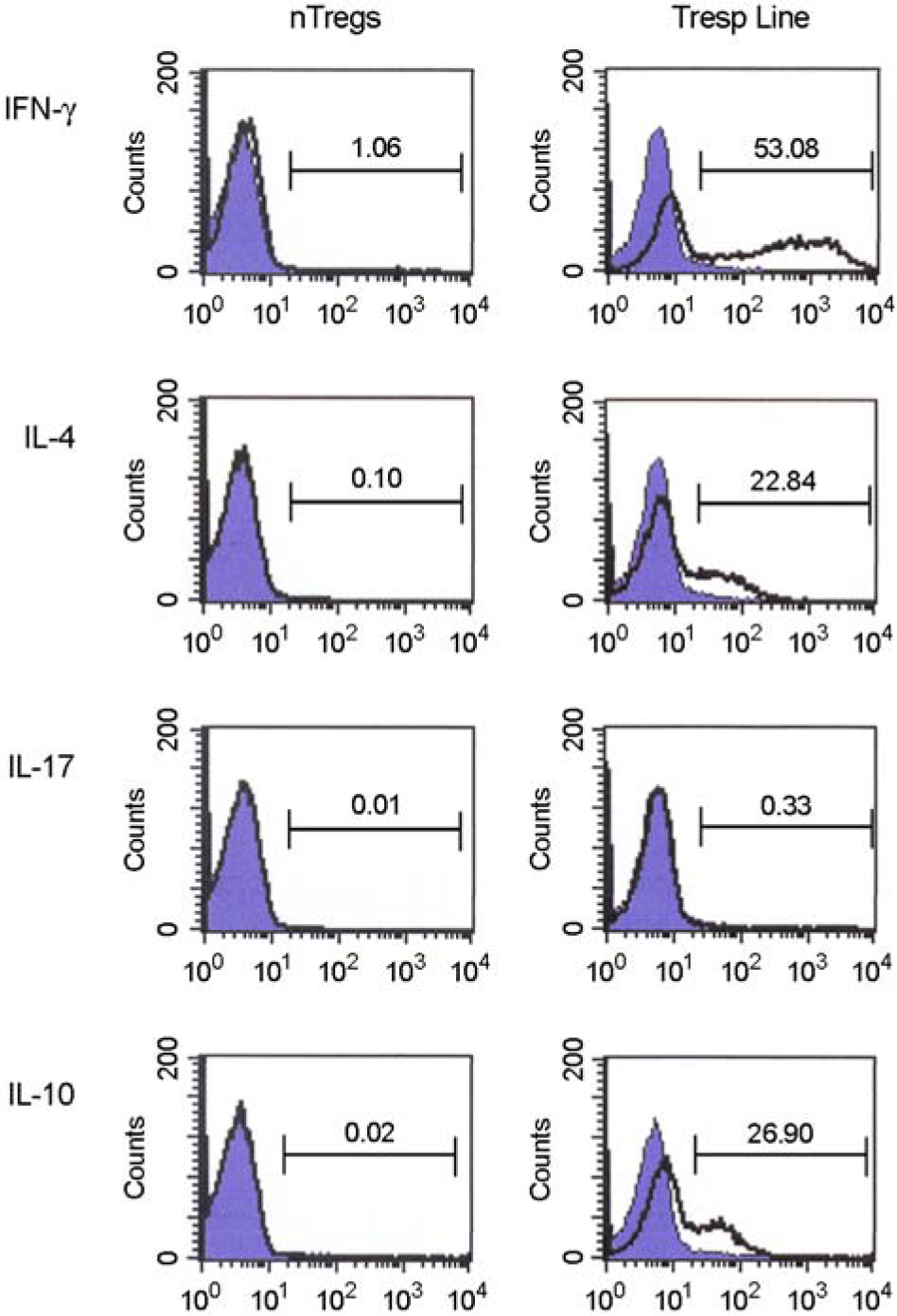
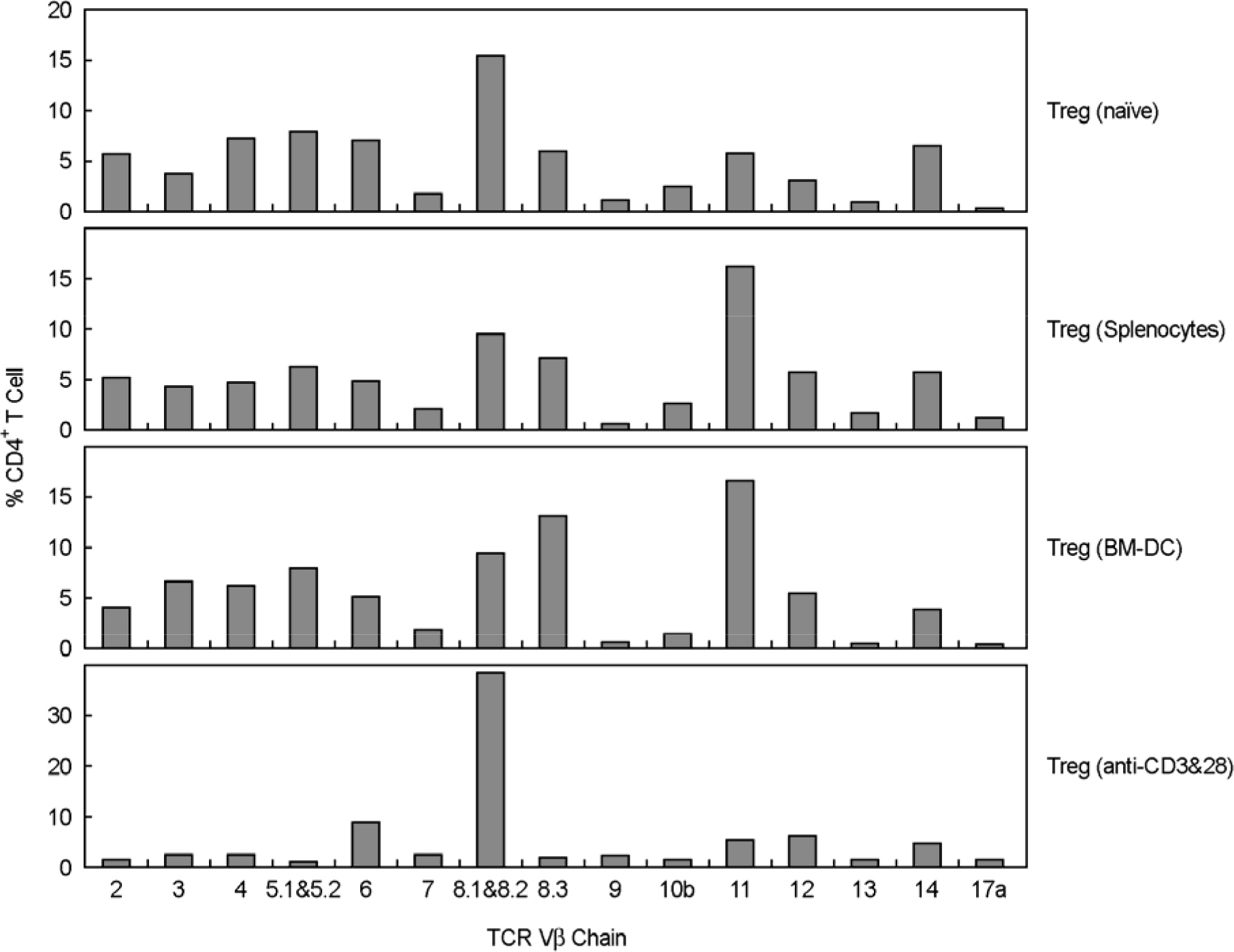
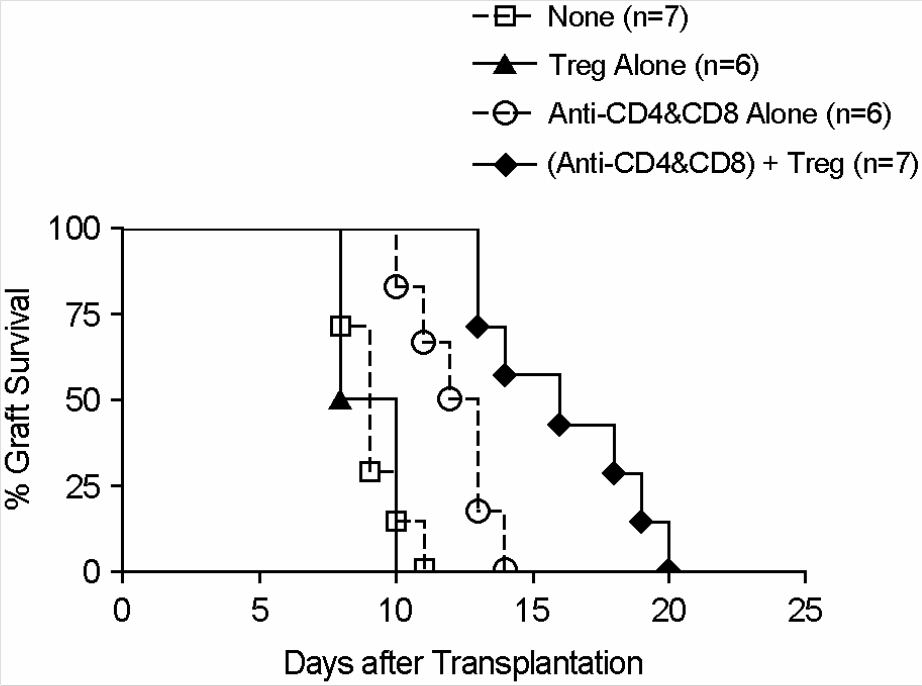
- Immunotherapy with regulatory T lymphocytes is considered to be an attractive new therapeutic modality to prevent allograft rejection. The success of this new therapy is critically dependent on the preparation of highly effective and enough number of regulatory T cells. Here, we tried to establish a proper strategy for the ex vivo expansion of regulatory T cells and evaluated their characteristics. CD4+CD25h+CD62L+ T cells were isolated from the recipient mice and weekly stimulated with various stimuli in the presence of IL-2. The most efficient protocol for the expansion of regulatory T cells maintaining Foxp3 expression and regulatory activity was the three cycles stimulation with donor bone marrow-derived dendritic cells (BM-DCs) which yielded around 400 fold expansion of regulatory T cells. The in vitro-expanded regulatory T cells expressing lymph node homing receptors on their cell surface, were composed of polyclonal population, and did not acquire the ability to produce effector cytokines. Importantly, these expanded regulatory T cells induced a modest prolongation of skin allograft survival when combined with transient T cell depletion in recipient mice. These data indicate that our protocol could be used to obtain an effective population of natural regulatory T cells available for the regulatory T cell therapy to prevent allograft rejection.
MeSH Terms
Figure
Reference
-
1). Dantal J., Soulillou JP. Immunosuppressive drugs and the risk of cancer after organ transplantation. N Engl J Med. 2005. 352:1371–3.
Article2). Lechler RI., Garden OA., Turka LA. The complementary roles of deletion and regulation in transplantation tolerance. Nat Rev Immunol. 2003. 3:147–58.
Article3). Zhang ZX., Yang L., Young KJ., DuTemple B., Zhang L. Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nat Med. 2000. 6:782–9.
Article4). Taylor PA., Noelle RJ., Blazar BR. CD4+CD25+ immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001. 193:1311–8.5). Hoffmann P., Ermann J., Edinger M., Fathman CG., Strober S. Donor-type CD4+CD25+ regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002. 196:389–99.6). Davies JD., O'Connor E., Hall D., Krahl T., Trotter J., Sarvetnick N. CD4+ CD45RB low-density cells from untreated mice prevent acute allograft rejection. J Immunol. 1999. 163:5353–7.7). Hara M., Kingsley CI., Niimi M., Read S., Turvey SE., Bushell AR., Morris PJ., Powrie F., Wood KJ. IL-10 is required for regulatory T cells to mediate tolerance to alloantigens in vivo. J Immunol. 2001. 166:3789–96.8). Ciubotariu R., Colovai AI., Pennesi G., Liu Z., Smith D., Berlocco P., Cortesini R., Suciu-Foca N. Specific suppression of human CD4+ TH-cell responses to pig MHC antigens by CD8+CD28– regulatory T cells. J Immunol. 1998. 161:5193–202.9). Bluestone JA., Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003. 3:253–7.
Article10). Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002. 2:389–400.11). Jonuleit H., Schmitt E. The regulatory T cell family: distinct subsets and their interrelations. J Immunol. 2003. 171:6323–7.
Article12). Daley SR., Ma J., Adams E., Cobbold SP., Waldmann H. A key role for TGF-β signaling to T cells in the long-term acceptance of allografts. J Immunol. 2007. 179:3648–54.
Article13). Carvalho-Gaspar M., Jones ND., Luo S., Martin L., Brook MO., Wood KJ. Location and time-dependent control of rejection by regulatory T cells culminates in a failure to generate memory T cells. J Immunol. 2008. 180:6640–8.
Article14). Wan YY., Flavell RA. The roles for cytokines in the generation and maintenance of regulatory T cells. Immunol Rev. 2006. 212:114–30.
Article15). Takahashi T., Kuniyasu Y., Toda M., Sakaguchi N., Itoh M., Iwata M., Shimizu J., Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998. 10:1969–80.16). Taylor PA., Lees CJ., Blazar BR. The infusion of ex vivo activated and expanded CD4+CD25+ immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002. 99:3493–9.17). Yamazaki S., Iyoda T., Tarbell K., Olson K., Velinzon K., Inaba K., Steinman RM. Direct expansion of functional CD25+CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003. 198:235–47.18). Tang Q., Henriksen KJ., Bi M., Finger EB., Szot G., Ye J., Masteller EL., McDevitt H., Bonyhadi M., Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004. 199:1455–65.19). Tarbell KV., Yamazaki S., Olson K., Toy P., Steinman RM. CD25+CD4+ T cells, expanded with dendritic cells presenting a single autoantigenic peptide, suppress autoimmune diabetes. J Exp Med. 2004. 199:1467–77.20). Chai JG., Coe D., Chen D., Simpson E., Dyson J., Scott D. In vitro expansion improves in vivo regulation by CD4+CD25+ regulatory T cells. J Immunol. 2008. 180:858–69.21). Sanchez-Fueyo A., Sandner S., Habicht A., Mariat C., Kenny J., Degauque N., Zheng XX., Strom TB., Turka LA., Sayegh MH. Specificity of CD4+CD25+ regulatory T cell function in alloimmunity. J Immunol. 2006. 176:329–34.22). Peters JH., Hilbrands LB., Koenen HJ., Joosten I. Ex vivo generation of human alloantigen-specific regulatory T cells from CD4posCD25high T cells for immunotherapy. PLoS One. 2008. 3:e2233.23). Huehn J., Siegmund K., Lehmann JC., Siewert C., Haubold U., Feuerer M., Debes GF., Lauber J., Frey O., Przybylski GK., Niesner U., de la Rosa M., Schmidt CA., Brauer R., Buer J., Scheffold A., Hamann A. Developmental stage, phenotype, and migration distinguish naïve- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004. 199:303–13.24). Suchin EJ., Langmuir PB., Palmer E., Sayegh MH., Wells AD., Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001. 166:973–81.25). Shevach EM. Mechanisms of Foxp3+ T regulatory cell-mediated suppression. Immunity. 2009. 30:636–45.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Proliferation of CD4(+)CD25(high+)Foxp3(+) regulatory T lymphocytes in ex vivo expanded ascitic fluid from primary and recurrent ovarian carcinoma
- Regulatory T Cells in B Cell Follicles
- Unusual Suspects in the Development of Obesity-Induced Inflammation and Insulin Resistance: NK cells, iNKT cells, and ILCs
- Regulatory T Cells and Infectious Disease
- Development of NK cell expansion methods using feeder cells from human myelogenous leukemia cell line