J Korean Soc Spine Surg.
2011 Dec;18(4):179-185. 10.4184/jkss.2011.18.4.179.
Development of an Intervertebral Disc Degeneration Model using Newzealand White Rabbits
- Affiliations
-
- 1Department of Orthopedic Surgery, Kwangju Christian Hospital, Gwangju, Korea. stemcellchoi@yahoo.co.kr
- 2Department of Orthopedic Surgery, Oregon Health and Science University, USA.
- KMID: 1447994
- DOI: http://doi.org/10.4184/jkss.2011.18.4.179
Abstract
- STUDY DESIGN: An experimental animal study.
OBJECTIVES
To create a more appropriate disc degeneration model which shows how Interleukin 1alpha may induce the activation of metalloproteinases within the nucleus pulposus. SUMMARY OF LITERATURE REVIEW: There are few disc degeneration models wherein there is activation of metalloproteinases within the nucleus pulposus without structural destruction of the intervertebral disc.
MATERIALS AND METHODS
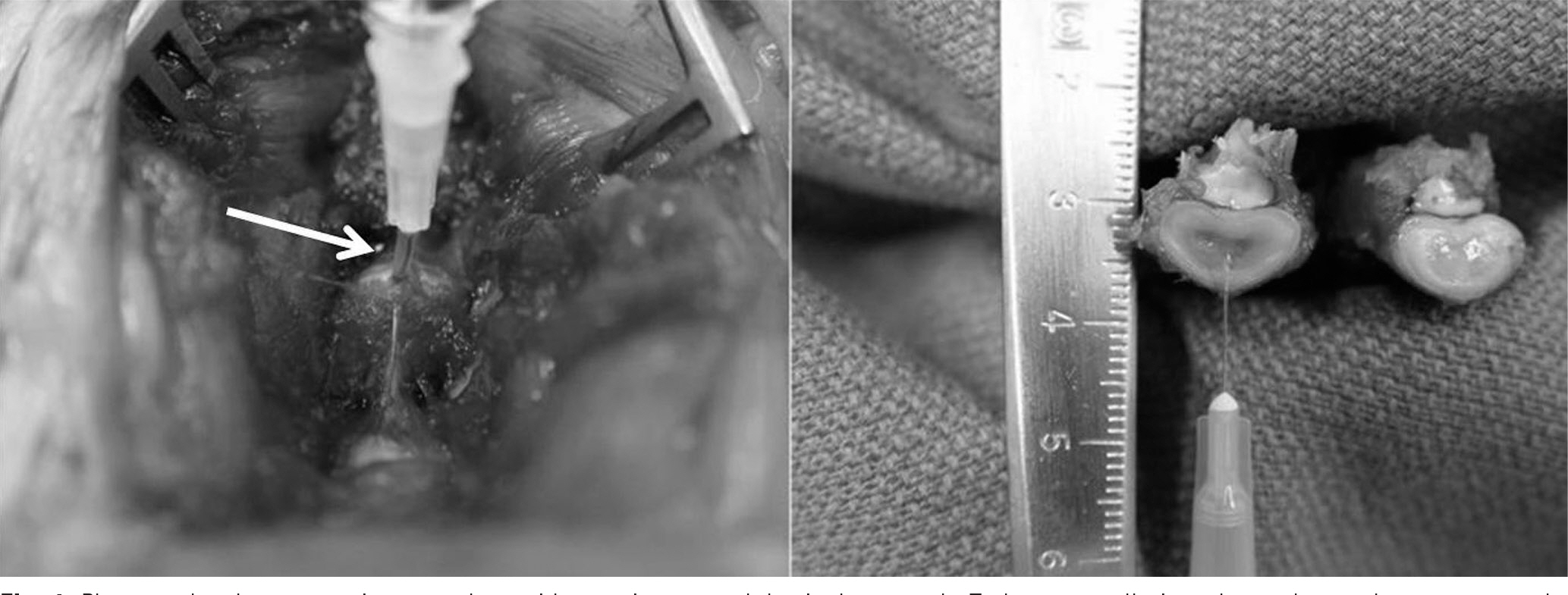
Three consecutive intervertebral discs in New Zealand White Rabbits were exposed. Each disc was injected with 0.1ml of saline (Saline group), 0.1ml of 1microg/ml (IL-1 group), 0.1ml of 10microg/ml (IL-10 group) of IL-1alpha through a 30-gauge needle. The lumbar spine was harvested 12 weeks after operation. We then analyzed radiographic findings and histological changes.
RESULTS
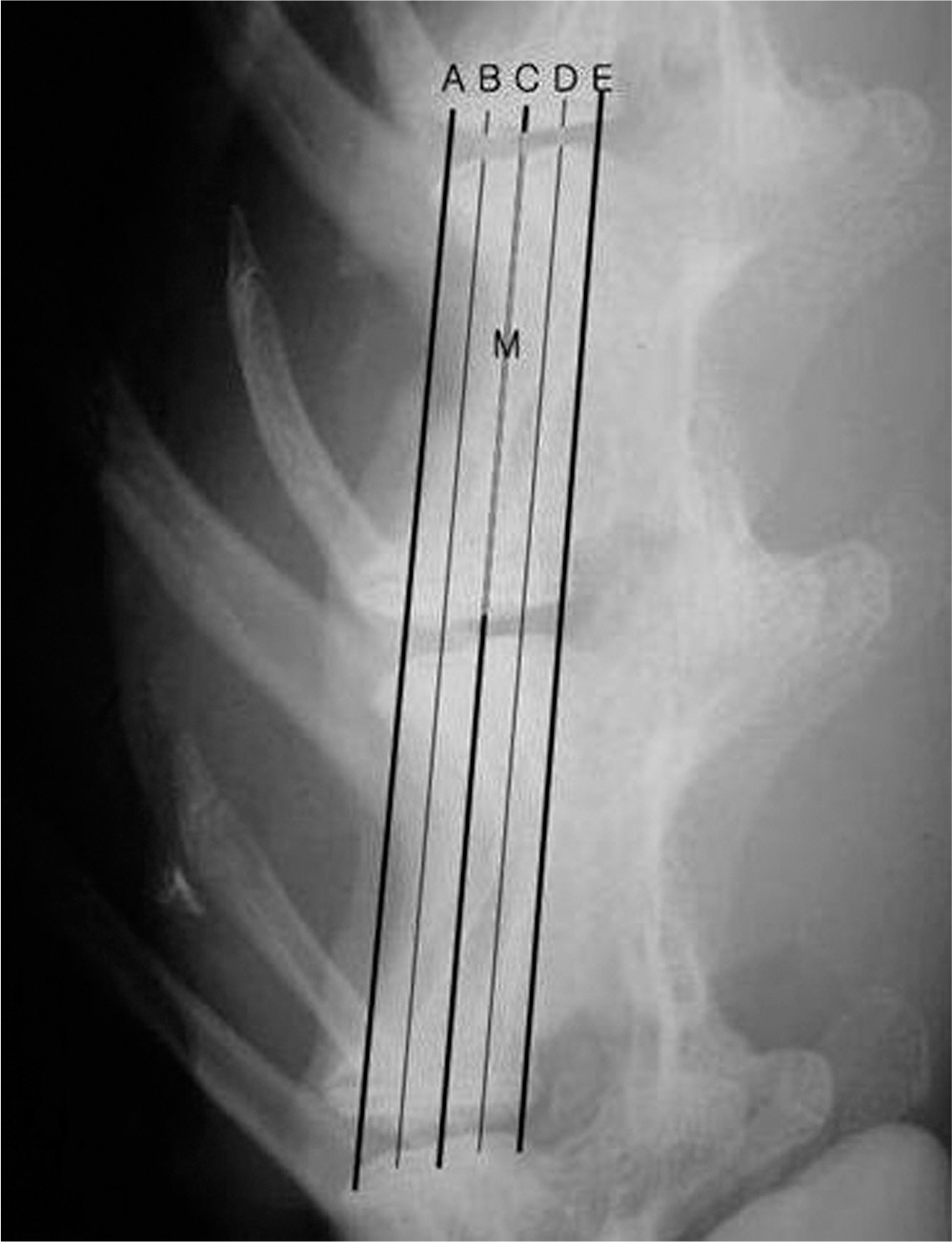
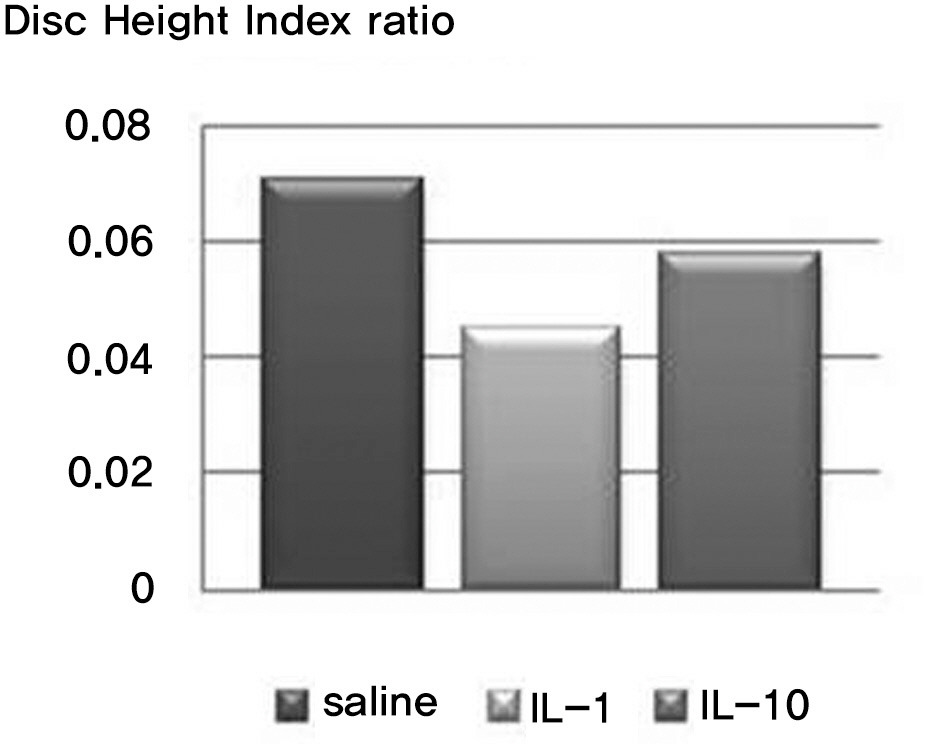
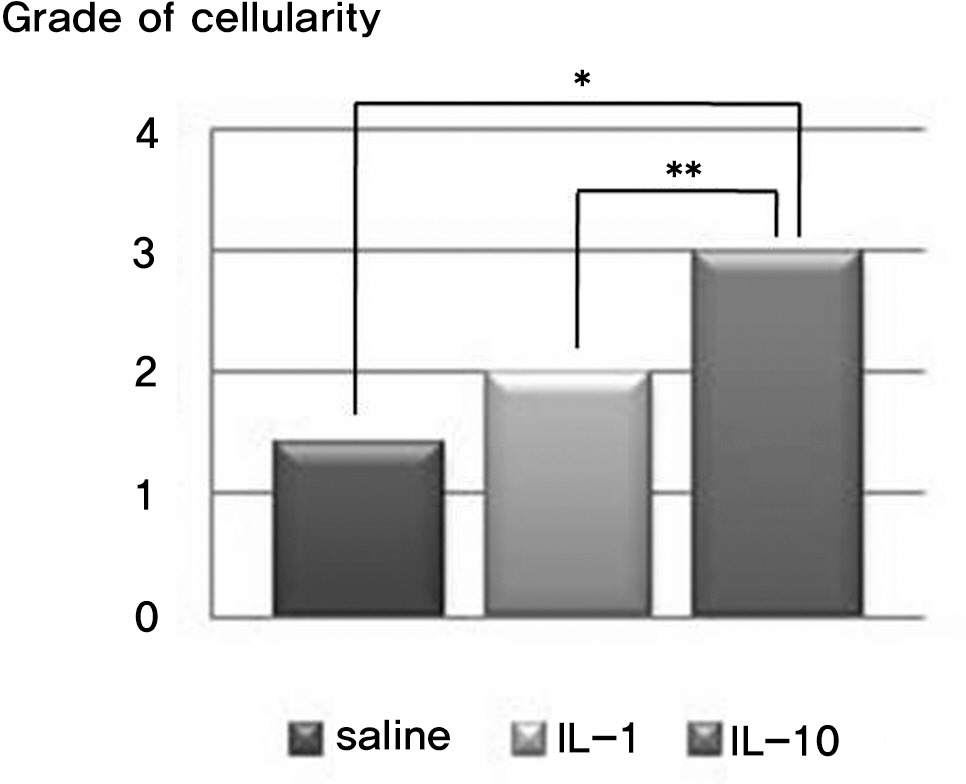
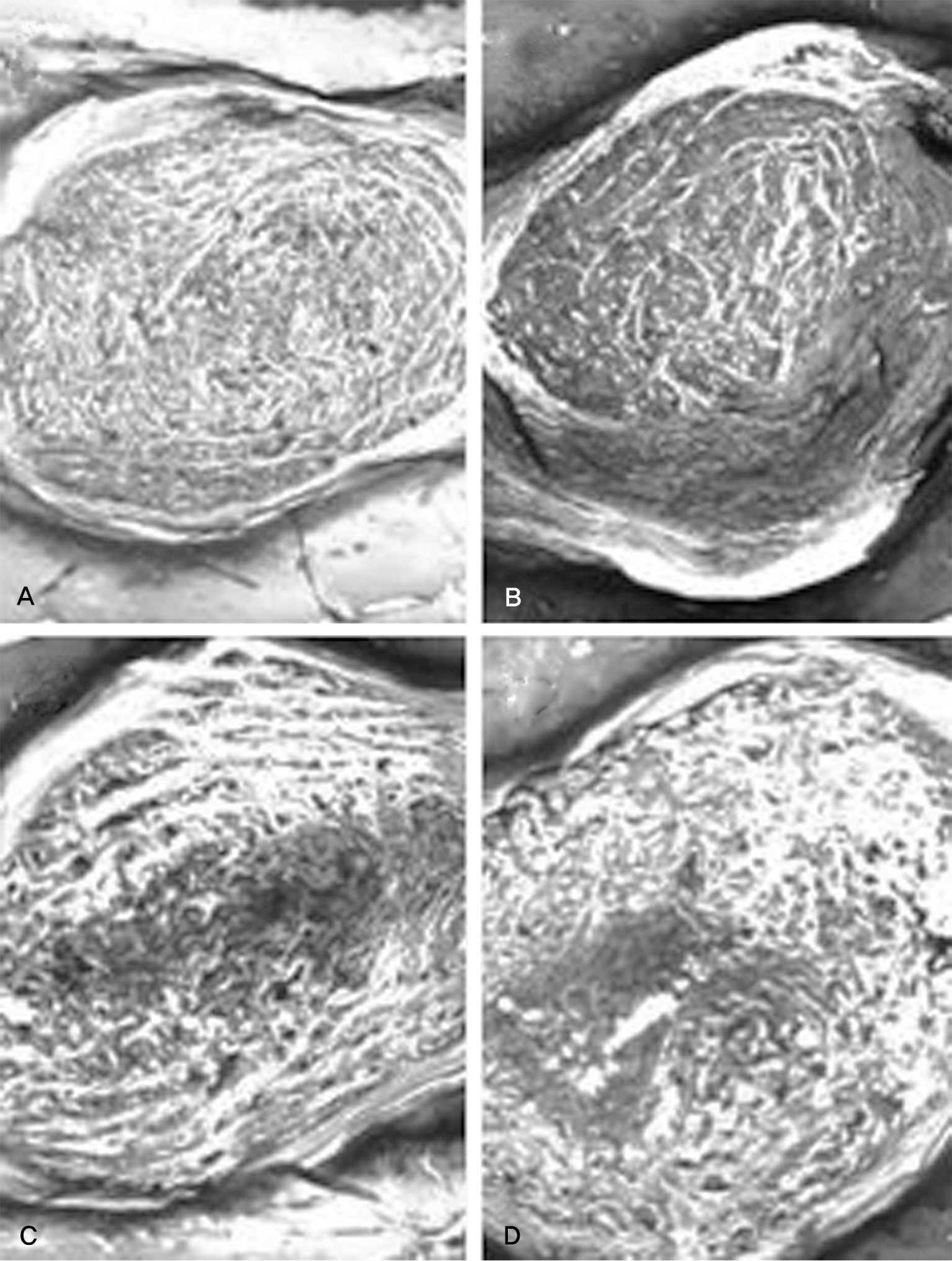
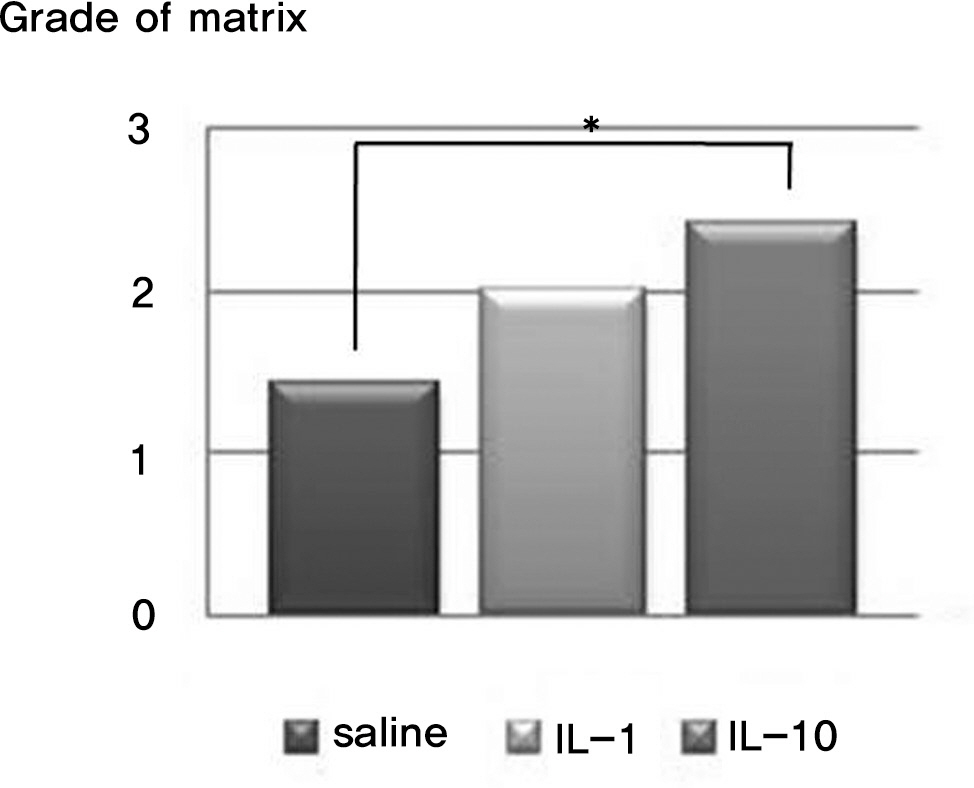
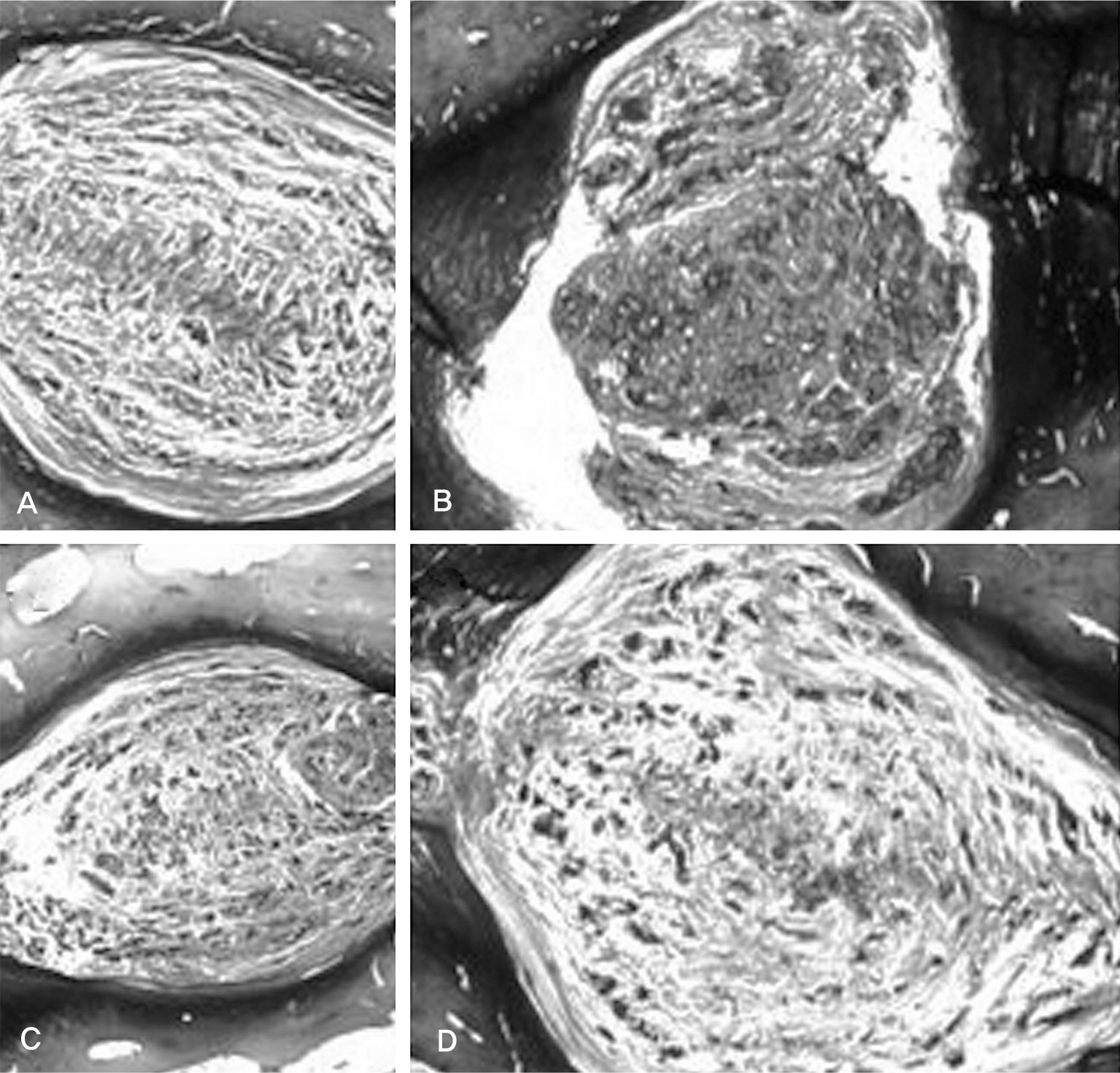
There was no difference in the radiological disc height index among the three groups; 0.071 in saline group, 0.045 in IL-1 group and 0.058 in IL-10 group (p=0.194). The histological cellularity of the nucleus pulposus revealed a decrease in the number of cells (p=0.0001, 1.42 in saline group vs. 3.00 in IL-10 group; p=0.001, 2.00 in IL-1 group and 3.00 in IL-10). The histological matrix of the nucleus pulposus was 1.42 in saline group and 2.42 in IL-10(p=0.007), which meant that there had been condensation of the extracellular nucleus pulposus matrix.
CONCLUSIONS
The results of this study demonstrate that interleukin-1alpha may contribute to degradation of the nucleus pulposus. This is useful for future study into the effects of the cytokine inhibitor on matrix regeneration and cellularity in the nucleus pulposus in intervertebral disc disease.
MeSH Terms
-
Animals
European Continental Ancestry Group
Humans
Interleukin-1
Interleukin-10
Interleukin-1alpha
Intervertebral Disc
Intervertebral Disc Degeneration
Intervertebral Disc Displacement
Metalloproteases
Needles
Rabbits
Regeneration
Spine
Interleukin-1
Interleukin-10
Interleukin-1alpha
Intervertebral Disc Degeneration
Intervertebral Disc Displacement
Metalloproteases
Figure
Reference
-
1. Deyo RA, Weinstein JN. Low back pain. N Engl J Med. 2001; 344:363–70.
Article2. Adams MA, Roughley PJ. What is Intervertebral Disc Degeneration, and What Causes It? Spine (Phila Pa 1976). 2006; 31:2151–61.
Article3. Takegami K, Thonar EJ, An HS, Kamada H, Masuda K. Osteogenic protein-1 enhances matrix replenishment by intervertebral disc cells previously exposed to interleukin-1. Spine (Phila Pa 1976). 2002; 27:1318–25.
Article4. An HS, Takegami K, Kamada H, et al. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine (Phila Pa 1976). 2005; 30:25–31.
Article5. Ganey T, Libera J, Moos V, et al. Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine (Phila Pa 1976). 2003; 28:2609–20.
Article6. Sakai D, Mochida J, Yamamoto Y, et al. Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials. 2003; 24:3531–41.
Article7. Sato M, Asazuma T, Ishihara M, et al. An experimental study of the regeneration of the intervertebral disc with an allograft of cultured annulus fibrosus cells using a tissue-engineering method. Spine (Phila Pa 1976). 2003; 28:548–53.
Article8. Smith JW, Walmsley R. Experimental incision of the intevertebral disc. J Bone Joint Surg. 1951; 33:612–25.9. Otani K, Arai I, Mao GP, Konno S, Olmarker K, Kikuchi S. Experimental disc herniation: evaluation of the natural course. Spine (Phila Pa 1976). 1997. 2894–9.10. Iatridis JC, Mente PL, Stokes LA, Aronsson DD, Alini M. Compression-induced changes in intervertebral disc properties in a rat tail model. Spine (Phila Pa 1976). 1999; 24:996–1002.
Article11. Sumida K, Sato K, Aoki M, Matsuyama Y, Iwata H. Serial changes in the rate of proteoglycan synthesis after chemonucleolysis of rabbit intervertebral discs. Spine (Phila Pa 1976). 1999; 24:1066–70.
Article12. Barksby HE, Hui W, Wappler I, et al. Interleukin-1 in combination with oncostatin M up-regulates multiple genes in chondrocytes: implications for cartilage destruction and repair. Arthritis Rheum. 2006; 54:540–50.
Article13. Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005; 7:732–45.14. Ghivizzani SC, Kang R, Georgescu HI, et al. Constitutive intra-articular expression of human IL-1 beta following gene transfer to rabbit synovium produces all major pathologies of human rheumatoid arthritis. J Immunol. 1997; 159:3604–12.15. Studer RK, Gilbertson LG, Georgescu H, Sowa G, Vo N, Kang JD. p38 MAPK inhibition modulates rabbit nucleus pulposus cell response to IL-1. J Orthop Res. 2008; 26:991–8.
Article16. Masuda K, Aota Y, Muehleman C, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine (Phila Pa 1976). 2005; 30:5–14.
Article17. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function and biochemistry. Circ Res. 2003; 92:827–39.18. Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Biol. 2004; 16:558–64.
Article19. Duffy MJ, Lynn DJ, Lloyd AT, O'Shea CM. The ADAMs family of proteins: From basic studies to proteinal clinical applications. Thromb Haemost. 2003; 89:622–31.20. Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005; 386:15–27.
Article21. Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanse: Their role in disorders of the human intervertebral disc. Spine (Phila Pa 1976). 2000; 25:3005–13.22. Goupille P, Jayson MI, Valat JP, Freemont AJ. Matrix metalloproteinases: The clue to intervertebral disc degeneration. Spine (Phila Pa 1976). 1998; 23:1612–26.
Article23. Antoniou J, Steffen T, Nelson F, et al. The human lumbar intervertebral disc: Evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996; 98:996–1003.
Article24. Doita M, Kanatani T, Harada T, Mizuno K. Immunohistologic study of the ruptured intervertebral disc of the lumbar spine. Spine (Phila Pa 1976). 1996; 21:235–41.
Article25. Burke JG, G Watson RW, Conhyea D, et al. Human nucleus pulposis can respond to a proinflammatory stimulus. Spine (Phila Pa 1976). 2003; 28:2685–93.
Article26. Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005; 7:732–45.27. Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine (Phila Pa 1976). 2002; 27:2631–44.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histological Changes after Intradiscal Steroid Injection to the Intervertebral Disc in Disc Injury Rabbit Model
- Biochemical Factors of Intervertebral Disc Degeneration: Implications for Disc Regeneration
- Effects of Growth Hormone on the Degenerative Changes in the Intervertebral Disc of Rabbits
- A Minimally Invasive Rabbit Model of Progressive and Reproducible Disc Degeneration Confirmed by Radiology, Gene Expression, and Histology
- Experimental Annulotomy-induced Degeneration in Rabbit Intervertebral Discs: Comparative Study Between Incomplete and Complete Annulotomy