J Korean Med Sci.
2017 Feb;32(2):343-351. 10.3346/jkms.2017.32.2.343.
Nestin Expression in the Adult Mouse Retina with Pharmaceutically Induced Retinal Degeneration
- Affiliations
-
- 1Department of Ophthalmology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea.
- 2Department of Ophthalmology, Hanyang University Guri Hospital, Guri, Korea.
- 3Department of Ophthalmology, Soonchunhyang University Bucheon Hospital, Bucheon, Korea. tkpark@schmc.ac.kr
- KMID: 2364181
- DOI: http://doi.org/10.3346/jkms.2017.32.2.343
Abstract
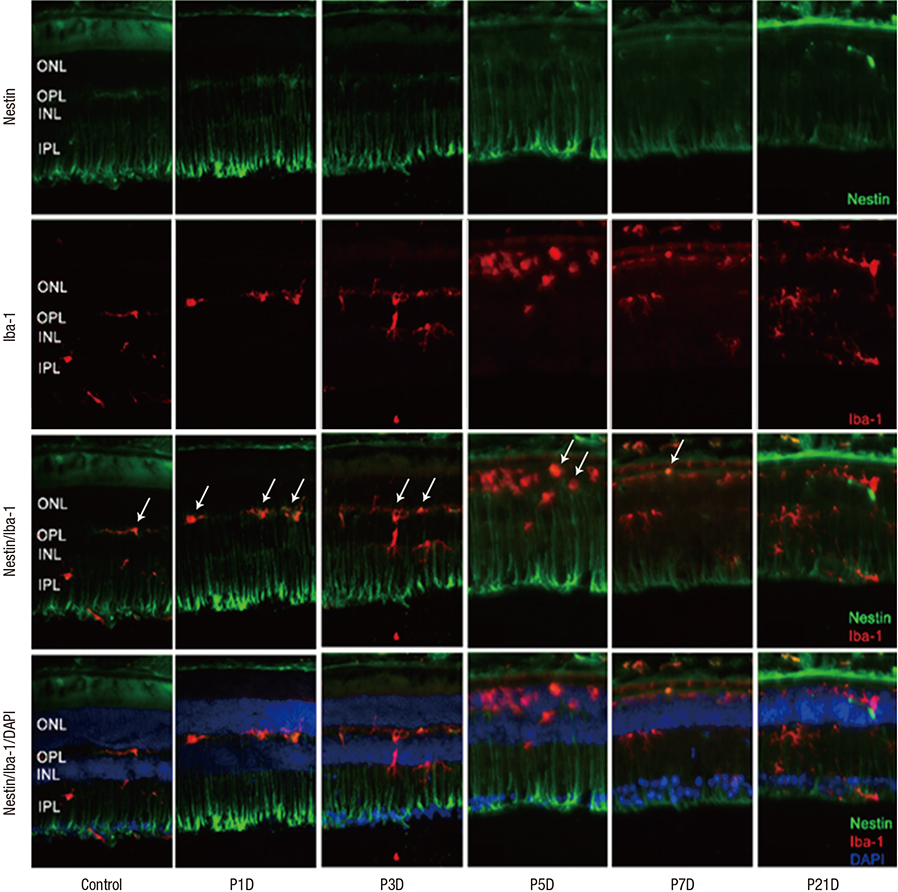
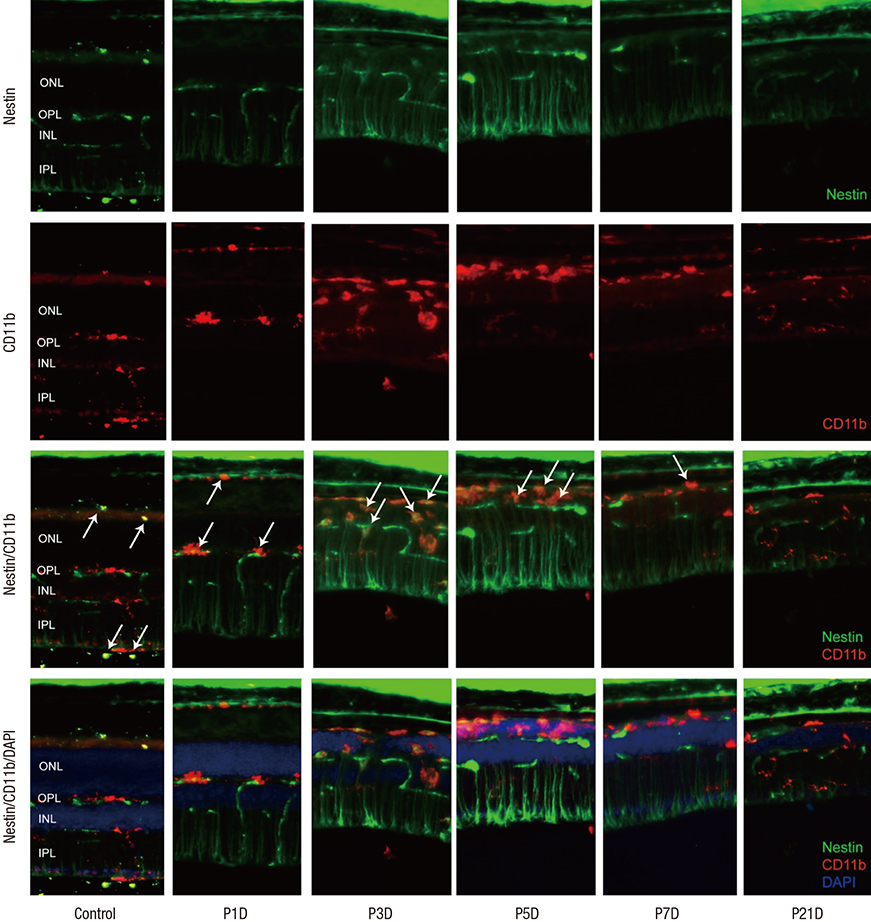
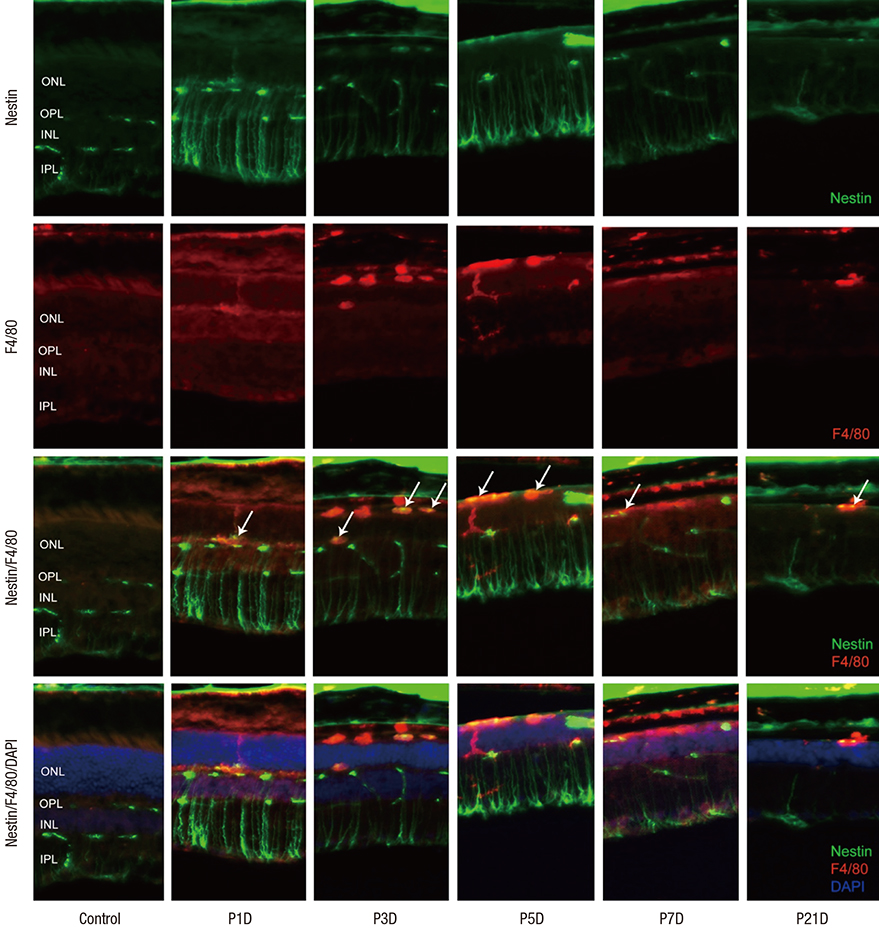
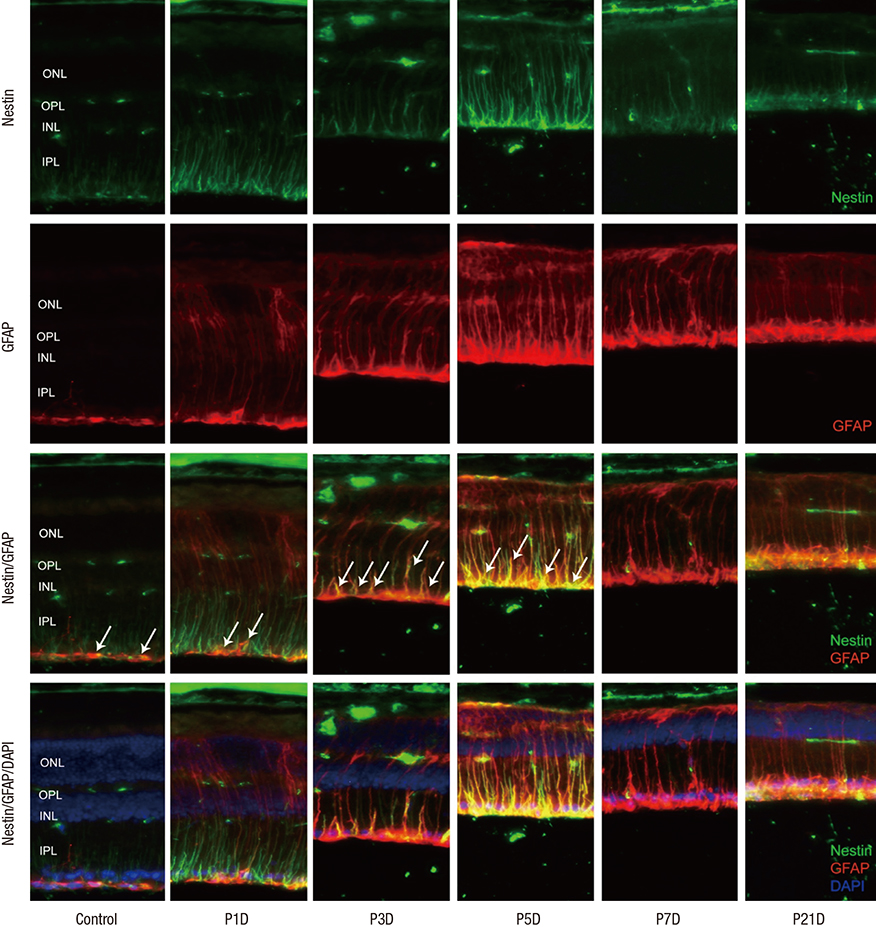
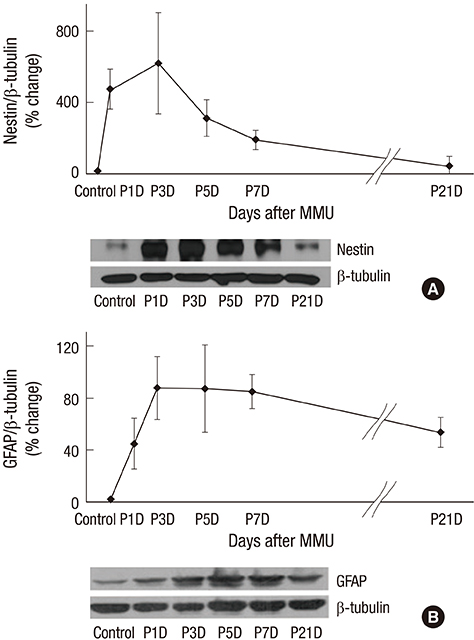
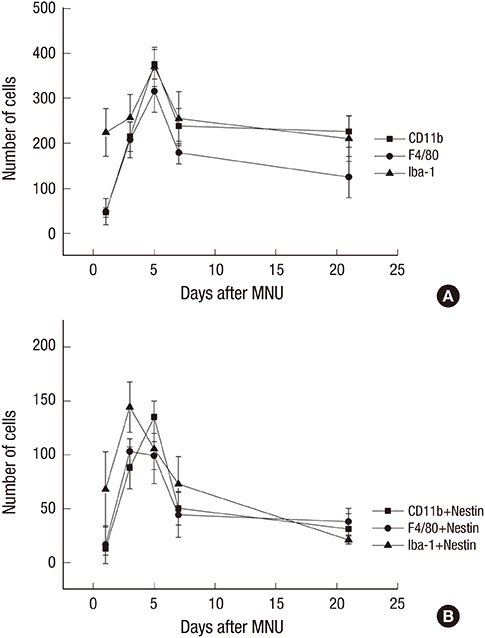
- The present study investigated the temporal pattern and cellular localization of nestin in the adult mouse retina with pharmaceutically induced retinal degeneration using N-methyl-N-nitrosourea (MNU). After a single intraperitoneal injection of MNU in 8-week-old C57BL/6 mice, the animals were sacrificed at 1, 3, 5, 7, and 21 days (n = 6, in each stage). The eyes were examined by means of immunohistochemical tests using nestin, ionized calcium-binding adaptor molecule (Iba-1), CD11b, F4/80, and glial fibrillary acidic protein (GFAP). Western blot analysis and manual cell counting were performed for quantification. Nestin expression was increased after MNU administration. Nestin+/Iba-1+ cells were migrated into outer nuclear layer (ONL) and peaked at day 3 post injection (PI). Nestin+/CD11b+ cells were also mainly identified in ONL at day 3 PI and peaked at day 5. Nestin+/F4/80+ cells were shown in the subretinal space and peaked at day 3 PI. Nestin+/GFAP+ cells were distinctly increased at day 1 PI and peaked at day 5 PI. The up-regulation of nestin expression after MNU administration in adult mouse retinal microglia, and monocyte/macrophage suggests that when retinal degeneration progresses, these cells may revert to a more developmentally immature state. Müller cells also showed reactive gliosis and differentiational changes.
MeSH Terms
Figure
Reference
-
1. Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990; 60:585–595.2. Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992; 255:1707–1710.3. Suzuki S, Namiki J, Shibata S, Mastuzaki Y, Okano H. The neural stem/progenitor cell marker nestin is expressed in proliferative endothelial cells, but not in mature vasculature. J Histochem Cytochem. 2010; 58:721–730.4. Dore-Duffy P, Katychev A, Wang X, Van Buren E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006; 26:613–624.5. Frisén J, Johansson CB, Török C, Risling M, Lendahl U. Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J Cell Biol. 1995; 131:453–464.6. Clarke SR, Shetty AK, Bradley JL, Turner DA. Reactive astrocytes express the embryonic intermediate neurofilament nestin. Neuroreport. 1994; 5:1885–1888.7. Walcott JC, Provis JM. Müller cells express the neuronal progenitor cell marker nestin in both differentiated and undifferentiated human foetal retina. Clin Experiment Ophthalmol. 2003; 31:246–249.8. Xue LP, Lu J, Cao Q, Hu S, Ding P, Ling EA. Müller glial cells express nestin coupled with glial fibrillary acidic protein in experimentally induced glaucoma in the rat retina. Neuroscience. 2006; 139:723–732.9. Wohl SG, Schmeer CW, Kretz A, Witte OW, Isenmann S. Optic nerve lesion increases cell proliferation and nestin expression in the adult mouse eye in vivo. Exp Neurol. 2009; 219:175–186.10. Kohno H, Sakai T, Kitahara K. Induction of nestin, Ki-67, and cyclin D1 expression in Müller cells after laser injury in adult rat retina. Graefes Arch Clin Exp Ophthalmol. 2006; 244:90–95.11. Wan J, Zheng H, Chen ZL, Xiao HL, Shen ZJ, Zhou GM. Preferential regeneration of photoreceptor from Müller glia after retinal degeneration in adult rat. Vision Res. 2008; 48:223–234.12. Luna G, Lewis GP, Banna CD, Skalli O, Fisher SK. Expression profiles of nestin and synemin in reactive astrocytes and Müller cells following retinal injury: a comparison with glial fibrillar acidic protein and vimentin. Mol Vis. 2010; 16:2511–2523.13. Wohl SG, Schmeer CW, Friese T, Witte OW, Isenmann S. In situ dividing and phagocytosing retinal microglia express nestin, vimentin, and NG2 in vivo. PLoS One. 2011; 6:e22408.14. Valamanesh F, Monnin J, Morand-Villeneuve N, Michel G, Zaher M, Miloudi S, Chemouni D, Jeanny JC, Versaux-Botteri C. Nestin expression in the retina of rats with inherited retinal degeneration. Exp Eye Res. 2013; 110:26–34.15. Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998; 57:1–9.16. London A, Itskovich E, Benhar I, Kalchenko V, Mack M, Jung S, Schwartz M. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. J Exp Med. 2011; 208:23–39.17. Caicedo A, Espinosa-Heidmann DG, Piña Y, Hernandez EP, Cousins SW. Blood-derived macrophages infiltrate the retina and activate Muller glial cells under experimental choroidal neovascularization. Exp Eye Res. 2005; 81:38–47.18. Erickson PA, Fisher SK, Guérin CJ, Anderson DH, Kaska DD. Glial fibrillary acidic protein increases in Müller cells after retinal detachment. Exp Eye Res. 1987; 44:37–48.19. Xue LP, Lu J, Cao Q, Kaur C, Ling EA. Nestin expression in Müller glial cells in postnatal rat retina and its upregulation following optic nerve transection. Neuroscience. 2006; 143:117–127.20. Xue L, Ding P, Xiao L, Hu M, Hu Z. Nestin, a new marker, expressed in Müller cells following retinal injury. Can J Neurol Sci. 2010; 37:643–649.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression Profiles of F4/80 and Nestin in Ocular Immune Cells Following Pharmaceutically Induced Retinal Degeneration in Adult Mice
- Expression of Nestin on Endothelial Cells and Pericytes During Retinal Vascular Development in Mouse
- Activation of Caspase-3 During Photoreceptor Degeneration in rd Mouse Retina
- Nestin expressing progenitor cells during establishment of the neural retina and its vasculature
- Experimental Degeneration of the Rabbits Retina by Sodium Glutamate