Korean J Physiol Pharmacol.
2013 Feb;17(1):9-13. 10.4196/kjpp.2013.17.1.9.
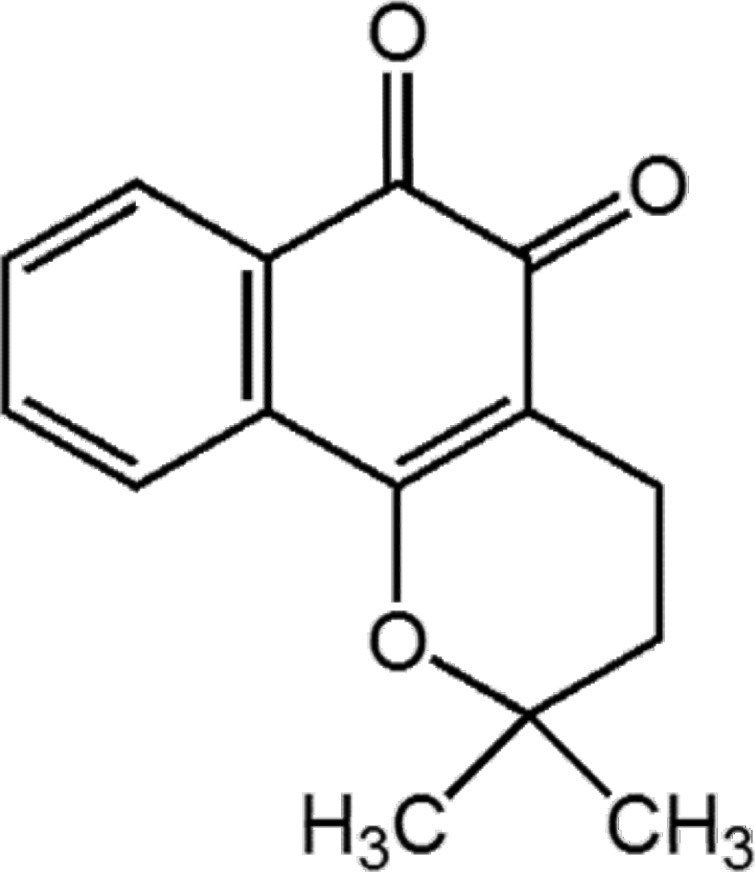
Impact of Micellar Vehicles on in situ Intestinal Absorption Properties of Beta-Lapachone in Rats
- Affiliations
-
- 1College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. jaehwi@cau.ac.kr
- 2Department of Pharmacology, College of Medicine, Chung-Ang University, Seoul 156-756, Korea.
- 3Department of Biomedical Science, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea.
- KMID: 1432757
- DOI: http://doi.org/10.4196/kjpp.2013.17.1.9
Abstract
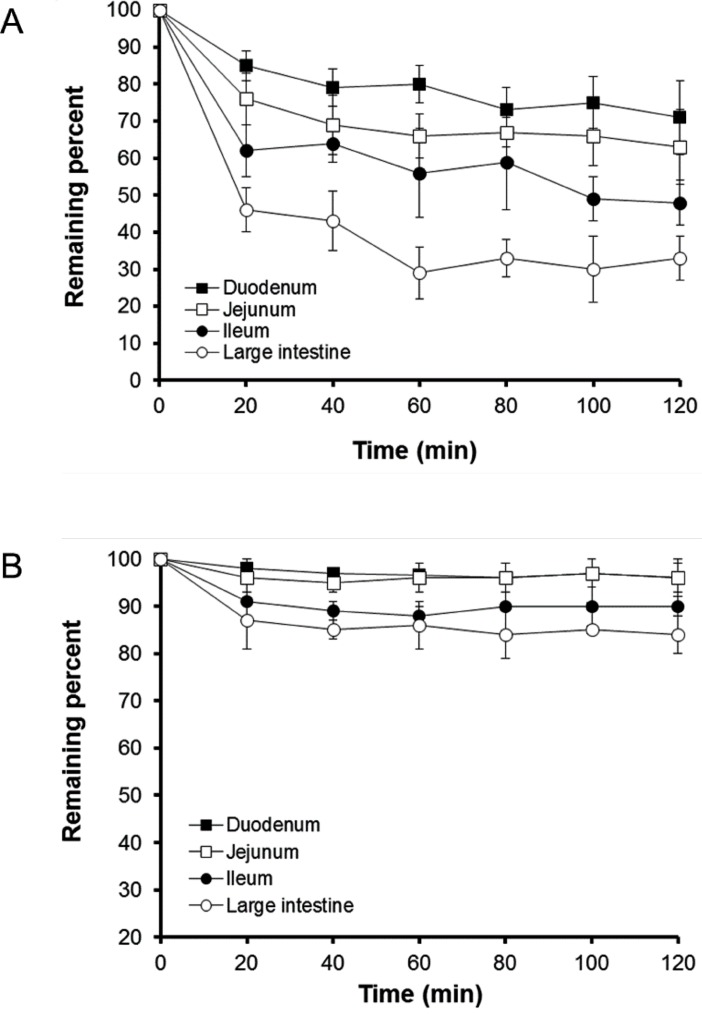
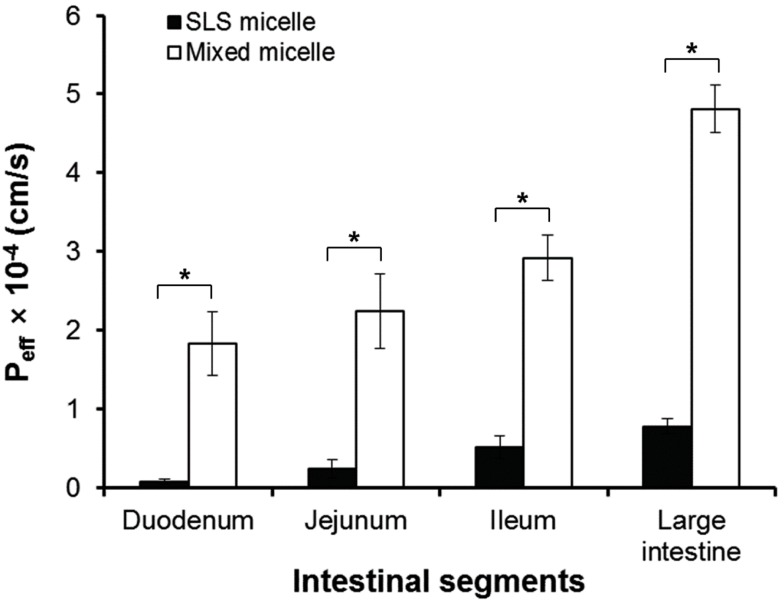
- The aim of the present study was to examine the effect of micellar systems on the absorption of beta-lapachone (b-lap) through different intestinal segments using a single-pass rat intestinal perfusion technique. B-lap was solubilized in mixed micelles composed of phosphatidylcholine and sodium deoxycholate, and in sodium lauryl sulfate (SLS)-based conventional micelles. Both mixed micelles and SLS micelles improved the in situ permeability of b-lap in all intestinal segments tested although the mixed micellar formulation was more effective in increasing the intestinal absorption of b-lap. The permeability of b-lap was greatest in the large intestinal segments. Compared with SLS micelles, the effective permeability coefficient values measured with mixed micelles were 5- to 23-fold higher depending on the intestinal segment. Our data suggest that b-lap should be delivered to the large intestine using a mixed micellar system for improved absorption.
MeSH Terms
Figure
Reference
-
1. Sakuma S, Tanno FK, Masaoka Y, Kataoka M, Kozaki T, Kamaguchi R, Kokubo H, Yamashita S. Effect of administration site in the gastrointestinal tracton bioavailability of poorly absorbed drugstaken after a meal. J Control Release. 2007; 118:59–64. PMID: 17250919.2. Persson EM, Nordgren A, Forsell P, Knutson L, Ohgren C, Forssén S, Lennernäs H, Abrahamsson B. Improved understanding of the effect of food on drug absorption and bioavailability for lipophilic compounds using an intestinal pig perfusion model. Eur J Pharm Sci. 2008; 34:22–29. PMID: 18387789.
Article3. Boyd BJ, Khoo SM, Whittaker DV, Davey G, Porter CJ. A lipid-based liquid crystalline matrix that provides sustained release and enhanced oral bioavailability for a model poorly water soluble drug in rats. Int J Pharm. 2007; 340:52–60. PMID: 17467935.
Article4. Sheen PC, Khetarpal VK, Cariola CM, Rowlings CE. Formulation studies of a poorly water-soluble drug in solid dispersions to improve bioavailability. Int J Pharm. 1995; 118:221–227.
Article5. Hauss DJ, Fogal SE, Ficorilli JV, Price CA, Roy T, Jayaraj AA, Keirns JJ. Lipid-based delivery systems for improving the bioavailability and lymphatic transport of a poorly water-soluble LTB4 inhibitor. J Pharm Sci. 1998; 87:164–169. PMID: 9519148.
Article6. McInnes GT, Asbury MJ, Ramsay LE, Shelton JR, Harrison IR. Effect of micronization on the bioavailability and pharmacologic activity of spironolactone. J Clin Pharmacol. 1982; 22:410–417. PMID: 7130430.
Article7. Mainardes RM, Urban MC, Cinto PO, Khalil NM, Chaud MV, Evangelista RC, Gremiao MP. Colloidal carriers for ophthalmic drug delivery. Curr Drug Targets. 2005; 6:363–371. PMID: 15857294.
Article8. O'Driscoll CM. Lipid-based formulations for intestinal lymphatic delivery. Eur J Pharm Sci. 2002; 15:405–415. PMID: 12036717.9. Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008; 83:761–769. PMID: 17957183.
Article10. Schiller WR. Summary of proceedings at the 43rd annual pancreas club meeting. J Gastrointest Surg. 2009; 13:2201–2211.
Article11. Noh JY, Park JS, Lim KM, Kim K, Bae ON, Chung SM, Shin S, Chung JH. A naphthoquinone derivative can induce anemia through phosphatidylserine exposure-mediated erythrophagocytosis. J Pharmacol Exp Ther. 2010; 333:414–420. PMID: 20164298.
Article12. Cunha-Filho MS, Dacunha-Marinho B, Torres-Labandeira JJ, Martínez-Pacheco R, Landín M. Characterization of betalapachone and methylated beta-cyclodextrin solid-state systems. AAPS PharmSciTech. 2007; 8:E60. PMID: 17915810.13. Li DX, Han MJ, Balakrishnan P, Yan YD, Oh DH, Joe JH, Seo Y, Kim JO, Park SM, Yong CS, Choi HG. Enhanced oral bioavailability of flurbiprofen by combined use of micelle solution and inclusion compound. Arch Pharm Res. 2010; 33:95–101. PMID: 20191350.
Article14. Yu JN, Zhu Y, Wang L, Peng M, Tong SS, Cao X, Qiu H, Xu XM. Enhancement of oral bioavailability of the poorly water-soluble drug silybin by sodium cholate/phospholipid-mixed micelles. Acta Pharmacol Sin. 2010; 31:759–764. PMID: 20523347.
Article15. des Rieux A, Fievez V, Garinot M, Schneider YJ, Préat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 2006; 116:1–27. PMID: 17050027.
Article16. Suresh D, Srinivasan K. Studies on the in vitro absorption of spice principles--curcumin, capsaicin and piperine in rat intestines. Food Chem Toxicol. 2007; 45:1437–1442. PMID: 17524539.17. Song NN, Li QS, Liu CX. Intestinal permeability of metformin using single-pass intestinal perfusion in rats. World J Gastroenterol. 2006; 12:4064–4070. PMID: 16810761.
Article18. Cook TJ, Shenoy SS. Intestinal permeability of chlorpyrifos using the single-pass intestinal perfusion method in the rat. Toxicology. 2003; 184:125–133. PMID: 12499115.
Article19. Kunes M, Svoboda Z, Květina J, Herout V, Herink J, Bajgar J. Intestinal single-pass in situ perfusion technique in rat: the influence of L-carnitine on absorption of 7-methoxytacrine. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2005; 149:433–435. PMID: 16601805.20. Liu J, Gong T, Wang C, Zhong Z, Zhang Z. Solid lipid nanoparticles loaded with insulin by sodium cholate-phosphatidylcholine-based mixed micelles: preparation and characterization. Int J Pharm. 2007; 340:153–162. PMID: 17428627.
Article21. Levitt MD, Furne JK, Levitt DG. Shaking of the intact rat and intestinal angulation diminish the jejunal unstirred layer. Gastroenterology. 1992; 103:1460–1466. PMID: 1426864.
Article22. Winne D, Görig H, Müller U. Closed rat jejunal segment in situ: role of pre-epithelial diffusion resistance (unstirred layer) in the absorption process and model analysis. Naunyn Schmiedebergs Arch Pharmacol. 1987; 335:204–215. PMID: 3561532.
Article23. Lindahl A, Sandström R, Ungell AL, Lennernäs H. Concentration-and region-dependent intestinal permeability of fluvastatin in the rat. J Pharm Pharmacol. 1998; 50:737–744. PMID: 9720622.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preclinical Pharmacokinetic Evaluation of beta-Lapachone: Characteristics of Oral Bioavailability and First-Pass Metabolism in Rats
- The possibility of low isomerization of β-lapachone in the human body
- Intestinal Absorption of Fibrinolytic and Proteolytic Lumbrokinase Extracted from Earthworm, Eisenia andrei
- beta-Lapachone suppresses radiation-induced activation of nuclear factor-kappaB
- beta-lapachone-Induced Apoptosis of Human Gastric Carcinoma AGS Cells Is Caspase-Dependent and Regulated by the PI3K/Akt Pathway