Korean J Physiol Pharmacol.
2010 Apr;14(2):71-75. 10.4196/kjpp.2010.14.2.71.
Intestinal Absorption of Fibrinolytic and Proteolytic Lumbrokinase Extracted from Earthworm, Eisenia andrei
- Affiliations
-
- 1Department of Pharmacology, College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. udsohn@cau.ac.kr
- 2Central Research Institute, Shin Poong Pharm. Co. Ltd., Ansan 425-100, Korea.
- KMID: 1457671
- DOI: http://doi.org/10.4196/kjpp.2010.14.2.71
Abstract
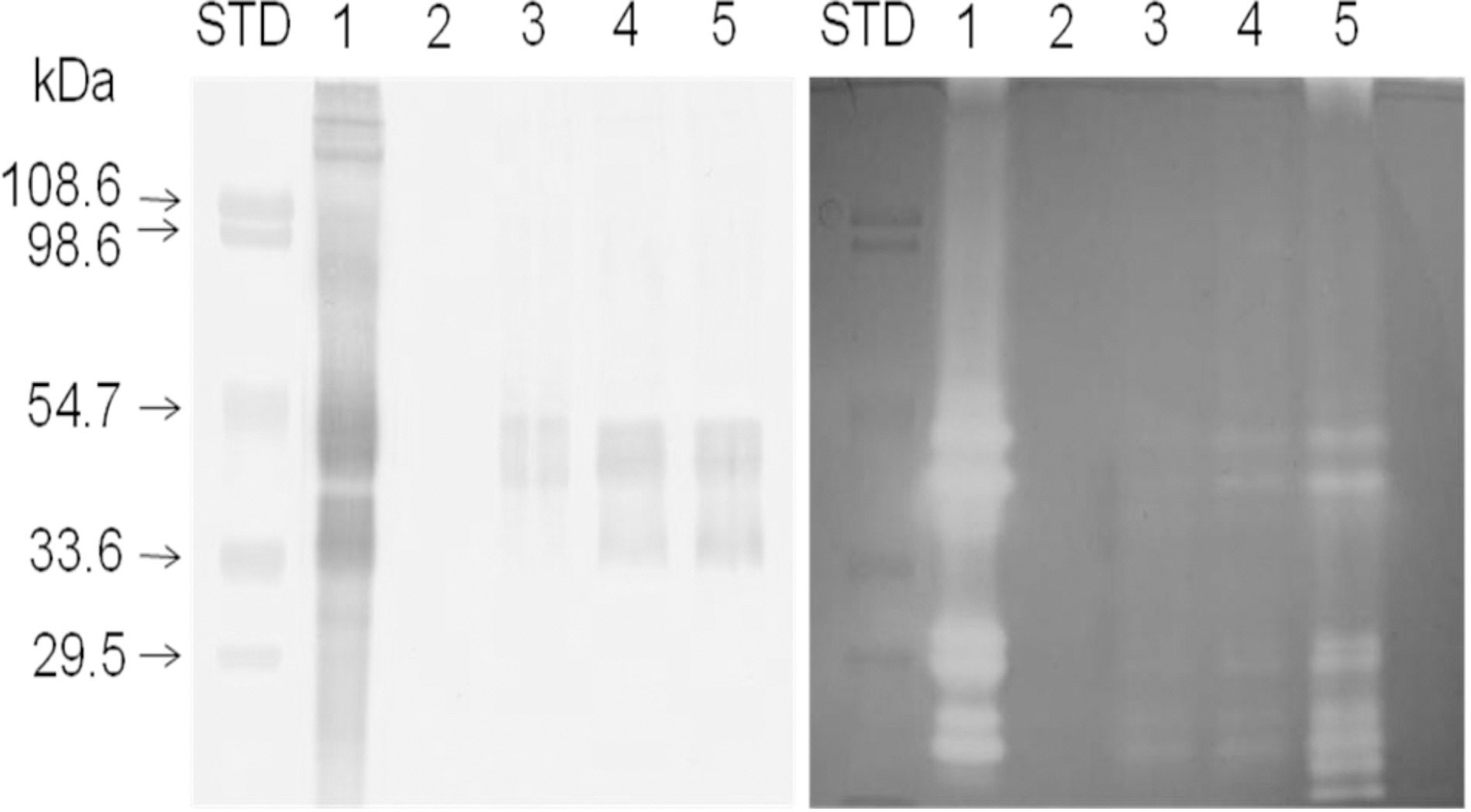
- To investigate the intestinal absorption of a fibrinolytic and proteolytic lumbrokinase extracted from Eisenia andrei, we used rat everted gut sacs and an in situ closed-loop recirculation method. We extracted lumbrokinase from Eisenia andrei, and then raised polyclonal antibody against lumbrokinase. Fibrinolytic activity and proteolytic activity in the serosal side of rat everted gut sacs incubated with lumbrokinase showed dose- and time-dependent patterns. Immunological results obtained by western blotting serosal side solution using rat everted gut sacs method showed that lumbrokinase proteins between 33.6 and 54.7 kDa are absorbed mostly by the intestinal epithelium. Furthermore, MALDI-TOF mass spectrometric analysis of plasma fractions obtained by in situ recirculation method confirmed that lumbrokinase F1 is absorbed into blood. These results support the notion that lumbrokinase can be absorbed from mucosal lumen into blood by oral administration.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Mihara H, Sumi H, Yoneta T, Mizumoto H, Ikeda R, Seiki M, Maruyama M. A novel fibrinolytic enzyme extracted from the earthworm, lumbricus rubellus. Jpn J Physiol. 1991; 41:461–472.
Article2. Cho IH, Choi ES, Lim HG, Lee HH. Purification and characterization of six fibrinolytic serine-proteases from earthworm lumbricus rubellus. J Biochem Mol Biol. 2004; 37:199–205.
Article3. Castell JV, Friedrich G, Kuhn CS, Poppe GE. Intestinal absorption of undegraded proteins in men: Presence of bromelain in plasma after oral intake. Am J Physiol. 1997; 273:G139–146.
Article4. Fan Q, Wu C, Li L, Fan R, Hou Q, He R. Some features of intestinal absorption of intact fibrinolytic enzyme iii-1 from lumbricus rubellus. Biochim Biophys Acta. 2001; 1526:286–292.
Article5. Fujita M, Hong K, Ito Y, Misawa S, Takeuchi N, Kariya K, Nishimuro S. Transport of nattokinase across the rat intestinal tract. Biol Pharm Bull. 1995; 18:1194–1196.
Article6. Vilhardt H, Lundin S. In vitro intestinal transport of vasopressin and its analogues. Acta Physiol Scand. 1986; 126:601–607.7. Lee CK, Shin JS, Kim BS, Cho IH, Kim YS, Lee EB. Antithrombotic effects by oral administration of novel proteinase fraction from earthworm eisenia andrei on venous thrombosis model in rats. Arch Pharm Res. 2007; 30:475–480.
Article8. Choi NS, Kim BY, Lee JY, Yoon KS, Han KY, Kim SH. Relationship between acrylamide concentration and enzymatic activity in an improve single fibrin zymogram gel system. J Biochem Mol Biol. 2002; 35:236–238.9. Zhou J, Fan R. Wu C, He RQ. Assay of lumbrokinase with a chromophoric substrate. Protein Pept Lett. 1997; 4:409–414.10. Kim SH, Choi NS, Lee WY. Fibrin zymography: a direct analysis of fibrinolytic enzymes on gels. Anal Biochem. 1998; 263:115–116.
Article11. Schanker LS, Tocco DJ, Brodie BB, Hogben CA. Absorption of drugs from the rat small intestine. J Pharmacol Exp Ther. 1958; 123:81–88.12. Mortz E, Krogh TN, Vorum H, Gorg A. Improved silver staining protocols for high sensitivity protein identification using matrix-assisted laser desorption/ionization-time of flight analysis. Proteomics. 2001; 1:1359–1363.
Article13. Landry F, Lombardo CR, Smith JW. A method for application of samples to matrix-assisted laser desorption ionization time-of-flight targets that enhances peptide detection. Anal Biochem. 2000; 279:1–8.
Article14. Shevchenko A, Jensen ON, Podtelejnikov AV, Sagliocco F, Wilm M, Vorm O, Mortensen P, Boucherie H, Mann M. Linking genome and proteome by mass spectrometry: Large-scale identification of yeast proteins from two dimensional gels. Proc Natl Acad Sci USA. 1996; 93:14440–14445.
Article15. Nakajima N, Ishihara K, Sugimoto M, Sumi H, Mikuni K, Hamada H. Chemical modification of earthworm fibrinolytic enzyme with human serum albumin fragment and characterization of the protease as a therapeutic enzyme. Biosci Biotechnol Biochem. 1996; 60:293–300.
Article16. Gardner ML. Gastrointestinal absorption of intact proteins. Annu Rev Nutr. 1988; 8:329–350.
Article17. Sanderson IR, Walker WA. Uptake and transport of macro-molecules by the intestine: Possible role in clinical disorders (an update). Gastroenterology. 1993; 104:622–639.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The high dosage of earthworm (Eisenia andrei) extract decreases cell proliferation and neuroblast differentiation in the mouse hippocampal dentate gyrus
- The thrombolytic effect of lumbrokinase is not as potent as urokinase in a rabbit cerebral embolism model
- Thrombolytic Effect of Lumbrokinase in Rat Cerebral Thromboembolism Model: a preliminary study
- Studies on Fibrinolytic System Behavior in Women with Polycystic Ovary Syndrome
- The effect of 1 alpha hydroxycholecalciferol on the intestinal absorption rate of 45Ca in patients with chronic renal failure