Korean J Urol.
2007 Apr;48(4):363-370. 10.4111/kju.2007.48.4.363.
The Initial Experience with 3rd Generation Nephron-sparing Cryoablation for Renal Tumor
- Affiliations
-
- 1Department of Urology, College of Medicine, Korea University, Seoul, Korea. mdksh@ korea.ac.kr
- KMID: 1997101
- DOI: http://doi.org/10.4111/kju.2007.48.4.363
Abstract
- PURPOSE
We report here on our initial experience with 3rd generation nephron-sparing renal cryoablation, which is one of the minimal invasive nephron-sparing surgeries.
MATERIALS AND METHODS
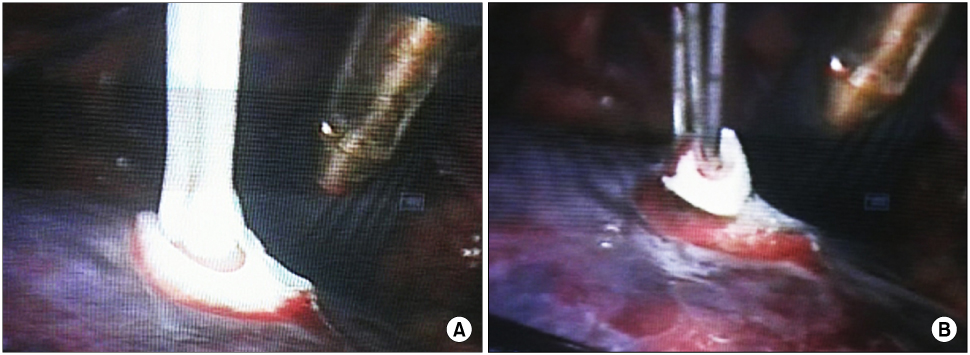
We enrolled 10 patients who had renal neoplasm identified on computed tomography (CT) and who underwent 3rd generation cryoablation from August 2004 to May 2006. The operative indications were a tumor less than 4cm in diameter, an exophytic mass with malignant potential and those cases like solitary kidney, bilateral tumor or renal insufficiency that needed preservation of renal function. Eight patients underwent minimal incision open cryoablation, and the other two underwent laparoscopic cryoablation. In every case, there were 2-3 cycles of freeze and thawing and we monitored the tumor and ice ball via intraoperative ultrasound.
RESULTS
The size of the iceball was maintained more than 1cm apart from the margin of the tumor to secure a safety margin. The average operation time of open cryoablation was 1.7 hr and that of laparoscopic cryoablation was 2.2 hr; the average blood loss was 194cc and 55cc, respectively. The average period of hospitalization after operation was 6.6 days and 3 days, respectively, and there was no complication except for one patient who had postoperative pulmonary effusion. During a mean follow-up of 14.1 months, no patient died and all the patients except one remained without local recurrence.
CONCLUSIONS
As a result of our initial experience, renal cryoablation turned out to have an excellent effect of tumor erradication and few complications. Although long term follow-up results are necessary, laparoscopic renal cryoablation is considered a minimal invasive nephron- sparing surgery that could be substituted for laparoscopic partial nephrectomy in the future.
Keyword
MeSH Terms
Figure
Reference
-
1. Gill IS, Novick AC, Soble JJ, Sung GT, Remer EM, Hale J, et al. Laparoscopic renal cryoablation: initial clinical series. Urology. 1998. 52:543–551.2. Bosniak MA, Birnbaum BA, Krinsky GA, Waisman J. Small renal parenchymal neoplasms: further observations on growth. Radiology. 1995. 197:589–597.3. Lau WK, Blute ML, Weaver AL, Torres VE, Zincke H. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc. 2000. 75:1236–1242.4. Oakley NE, Hegarty NJ, McNeill A, Gill IS. Minimally invasive nephron-sparing surgery for renal cell cancer. BJU Int. 2006. 98:278–284.5. Lawatsch EJ, Langenstroer P, Byrd GF, See WA, Quiroz FA, Begun FP. Intermediate results of laparoscopic cryoablation in 59 patients at the medical college of Wisconsin. J Urol. 2006. 175:1225–1229.6. Desai MM, Aron M, Gill IS. Laparoscopic partial nephrectomy versus laparoscopic cryoablation for the small renal tumor. Urology. 2005. 66(5 Suppl):23–28.7. Breining H, Helpap B, Minderjahn A, Lymberopoulos S. The parenchymal reaction of the kidney after local freezing. Urol Res. 1974. 2:29–31.8. Uchida M, Imaide Y, Sugimoto K, Uehara H, Watanabe H. Percutaneous cryosurgery for renal tumours. Br J Urol. 1995. 75:132–136.9. Moinzadeh A, Spaliviero M, Gill IS. Cryotherapy of renal masses: intermediate-term follow-up. J Endourol. 2005. 19:654–657.10. Wyler SF, Sulser T, Ruszat R, Weltzien B, Forster TH, Provenzano M, et al. Intermediate-term results of retroperitoneoscopy-assisted cryotherapy for small renal tumours using multiple ultrathin cryoprobes. Eur Urol. 2007. 51:971–979.11. Gill IS, Remer EM, Hasan WA, Strzempkowski B, Spaliviero M, Steinberg AP, et al. Renal cryoablation: outcome at 3 years. J Urol. 2005. 173:1903–1907.12. Davol PE, Fulmer BR, Rukstalis DB. Long-term results of cryoablation for renal cancer and complex renal masses. Urology. 2006. 68:2–6.13. Gill IS. Renal cryotherapy: pro. Urology. 2005. 65:415–418.14. Chosy SG, Nakada SY, Lee FT Jr, Warner TF. Monitoring renal cryosurgery: predictors of tissue necrosis in swine. J Urol. 1998. 159:1370–1374.15. Campbell SC, Krishnamurthi V, Chow G, Hale J, Myles J, Novick AC. Renal cryosurgery: experimental evaluation of treatment parameters. Urology. 1998. 52:29–33.16. Sindelar WF, Javadpour N, Bagley DH. Histological and ultrastructural changes in rat kidney after cryosurgery. J Surg Oncol. 1981. 18:363–379.17. Theodorescu D. Cancer cryotherapy: evolution and biology. Rev Urol. 2004. 6:Suppl 4. S9–S19.18. Cestari A, Guazzoni G, dell'Acqua V, Nava L, Cardone G, Balconi G, et al. Laparoscopic cryoablation of solid renal masses: intermediate term followup. J Urol. 2004. 172:1267–1270.19. Lee DI, Clayman RV. Percutaneous approaches to renal cryoablation. J Endourol. 2004. 18:643–646.20. Gill IS, Novick AC, Soble JJ, Sung GT, Remer EM, Hale J, et al. Laparoscopic renal cryoablation: initial clinical series. Urology. 1998. 52:543–551.21. Byrd GF, Lawatsch EJ, Mesrobian HG, Begun F, Langenstroer P. Laparoscopic cryoablation of renal angiomyolipoma. J Urol. 2006. 176:1512–1516.22. Yip SK, Tan PH, Cheng WS, Li MK, Foo KT. Surgical management of angiomyolipoma: nephron-sparing surgery for symptomatic tumour. Scand J Urol Nephrol. 2000. 34:32–35.23. Richter F, Kasabian NG, Irwin RJ Jr, Watson RA, Lang EK. Accuracy of diagnosis by guided biopsy of renal mass lesions classified indeterminate by imaging studies. Urology. 2000. 55:348–352.24. Sung GT, Gill IS, Hsu TH, Meraney AM, Skacel M, Brainard JA, et al. Effect of intentional cryoinjury to the renal collecting system. J Urol. 2003. 170:619–622.25. Janzen NK, Perry KT, Han KR, Kristo B, Raman S, Said JW, et al. The effects of intentional cryoablation and radio frequency ablation of renal tissue involving the collecting system in a porcine model. J Urol. 2005. 173:1368–1374.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Laparoscopic Nephron Sparing Surgery in Renal Tumor
- Patterns of Tumor Recurrence after Nephron Sparing Surgery for Renal Cell Carcinoma
- Nephron Sparing Surgery Using Expanded Polytetrafluoroethylene
- Clinical Experience of Nephron Sparing Surgery for Renal Tumor with a Normal Opposite Kidney
- Laparoscopic Nephron Sparing Surgery for Small Renal Cell Carcinoma less than 4cm