Korean J Radiol.
2010 Dec;11(6):618-626. 10.3348/kjr.2010.11.6.618.
Molecularly Targeted Therapy Using Bevacizumab for Non-Small Cell Lung Cancer: a Pilot Study for the New CT Response Criteria
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea. kyungs.lee@samsung.com
- 2Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea.
- KMID: 1119224
- DOI: http://doi.org/10.3348/kjr.2010.11.6.618
Abstract
OBJECTIVE
We wanted to compare the efficacy of the new CT response evaluation criteria for predicting the tumor progression-free survival (PFS) with that of RECIST 1.1 in non-small cell lung cancer (NSCLC) patients who were treated with bevacizumab.
MATERIALS AND METHODS
Sixteen patients (M:F = 11:5; median age, 57 years) treated with bevacizumab and combined cytotoxic chemotherapeutic agents were selected for a retrospective analysis. The tumor response was assessed by four different methods, namely, by using RECIST 1.1 (RECIST), RECIST but measuring only the solid component of tumor (RECISTsolid), the alternative method reflecting tumor cavitation (the alternative method) and the combined criteria (the combined criteria) that evaluated both the changes of tumor size and attenuation. To evaluate the capabilities of the different measurement methods to predict the patient prognosis, the PFS were compared, using the log rank test, among the responder groups (complete response [CR], partial response [PR], stable disease [SD] and progressive disease [PD]) in terms of the four different methods.
RESULTS
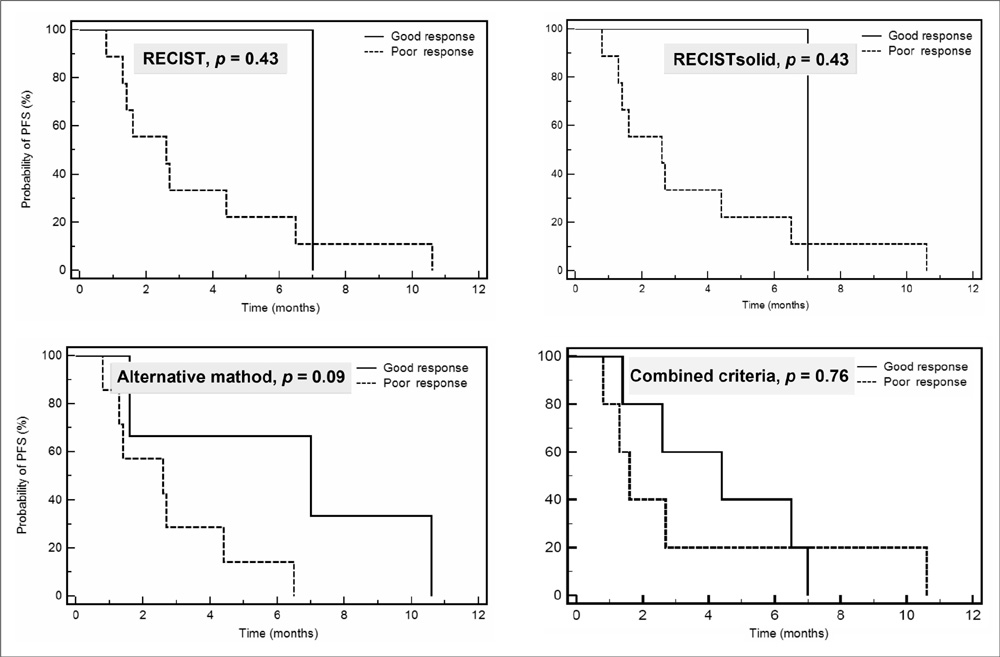
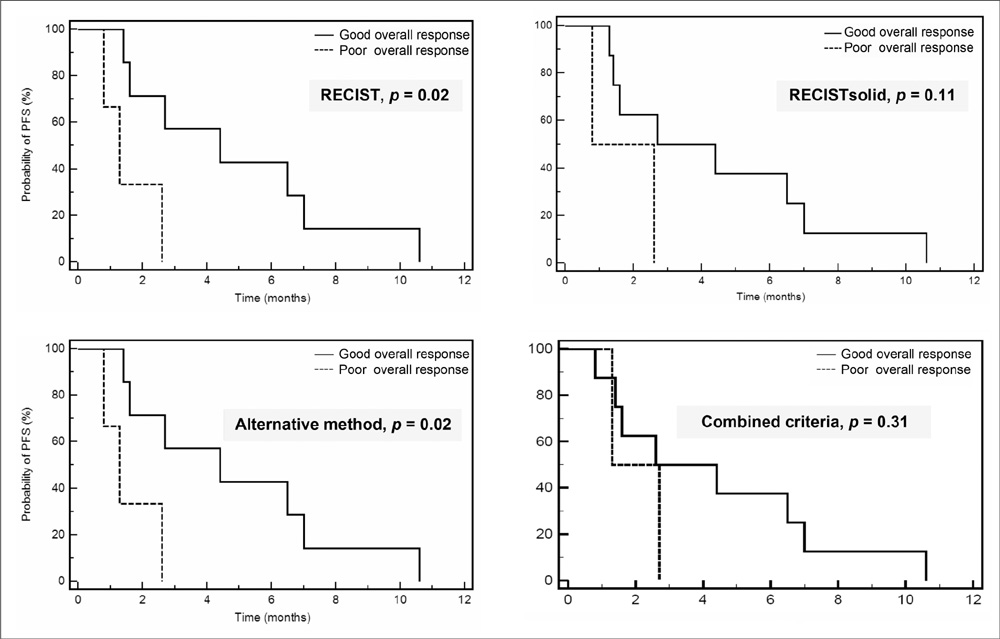
The overall (CR, PR or SD) response rates according to RECIST, RECISTsolid, the alternative method and the combined criteria were 81%, 88%, 81% and 85%, respectively. The confirmed response rates (CR or PR) were 19%, 19%, 50% and 54%, respectively. Although statistically not significant, the alternative method showed the biggest difference for predicting PFS among the three response groups (PR, SD and PD) (p = 0.07). RECIST and the alternative method showed a significant difference for predicting the prognosis between the good (PR or SD) and poor overall responders (p = 0.02).
CONCLUSION
The response outcome evaluations using the three different CT response criteria that reflect tumor cavitation, the ground-glass opacity component and the attenuation changes in NSCLC patients treated with bevacizumab showed different results from that with using the traditional RECIST method.
Keyword
MeSH Terms
-
Angiogenesis Inhibitors/*therapeutic use
Antibodies, Monoclonal/*therapeutic use
Carcinoma, Non-Small-Cell Lung/*drug therapy/pathology/*radiography
Disease Progression
Female
Humans
Lung Neoplasms/*drug therapy/pathology/*radiography
Male
Middle Aged
Pilot Projects
Prognosis
Radiographic Image Interpretation, Computer-Assisted
Retrospective Studies
Salvage Therapy
Survival Rate
*Tomography, X-Ray Computed
Treatment Outcome
Figure
Cited by 1 articles
-
Comparison of the Diagnostic Performance of Response Evaluation Criteria in Solid Tumor 1.0 with Response Evaluation Criteria in Solid Tumor 1.1 on MRI in Advanced Breast Cancer Response Evaluation to Neoadjuvant Chemotherapy
Su Kyung Jeh, Sung Hun Kim, Bong Joo Kang
Korean J Radiol. 2013;14(1):13-20. doi: 10.3348/kjr.2013.14.1.13.
Reference
-
1. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000. 92:205–216.2. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009. 45:228–247.3. Crabb SJ, Patsios D, Sauerbrei E, Ellis PM, Arnold A, Goss G, et al. Tumor cavitation: impact on objective response evaluation in trials of angiogenesis inhibitors in non-small-cell lung cancer. J Clin Oncol. 2009. 27:404–410.4. Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007. 25:1753–1759.5. Stacchiotti S, Collini P, Messina A, Morosi C, Barisella M, Bertulli R, et al. High-grade soft-tissue sarcomas: tumor response assessment--pilot study to assess the correlation between radiologic and pathologic response by using RECIST and Choi criteria. Radiology. 2009. 251:447–456.6. Lee HY, Han J, Lee KS, Koo JH, Jeong SY, Kim BT, et al. Lung adenocarcinoma as a solitary pulmonary nodule: prognostic determinants of CT, PET, and histopathologic findings. Lung Cancer. 2009. 66:379–385.7. Curran SD, Muellner AU, Schwartz LH. Imaging response assessment in oncology. Cancer Imaging. 2006. 6:S126–S130.8. Gwyther SJ. Current standards for response evaluation by imaging techniques. Eur J Nucl Med Mol Imaging. 2006. 33:11–15.9. Ratain MJ, Eckhardt SG. Phase II studies of modern drugs directed against new targets: if you are fazed, too, then resist RECIST. J Clin Oncol. 2004. 22:4442–4445.10. Sabir A, Schor-Bardach R, Wilcox CJ, Rahmanuddin S, Atkins MB, Kruskal JB, et al. Perfusion MDCT enables early detection of therapeutic response to antiangiogenic therapy. AJR Am J Roentgenol. 2008. 191:133–139.11. Kan Z, Phongkitkarun S, Kobayashi S, Tang Y, Ellis LM, Lee TY, et al. Functional CT for quantifying tumor perfusion in antiangiogenic therapy in a rat model. Radiology. 2005. 237:151–158.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Molecularly Targeted Therapy for Lung Cancer : Recent Topics
- Immunotherapy for Non-Small Cell Lung Cancer
- Challenges in the Use of Targeted Therapies in Non–Small Cell Lung Cancer
- New Targeted Therapy for Non-Small Cell Lung Cancer
- Targeted therapy and tailored chemotherapy for advanced non-small cell lung cancer