J Vet Sci.
2010 Mar;11(1):51-58. 10.4142/jvs.2010.11.1.51.
Antimutagenic and anticarcinogenic effect of methanol extracts of Petasites japonicus Maxim leaves
- Affiliations
-
- 1National Veterinary Research and Quarantine Service, Anyang 430-757, Korea.
- KMID: 1106157
- DOI: http://doi.org/10.4142/jvs.2010.11.1.51
Abstract
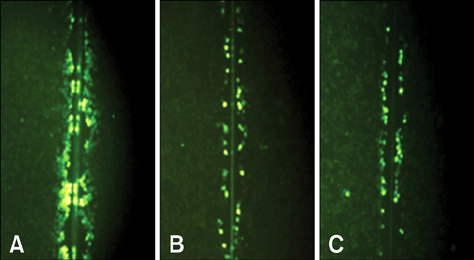
- The methanol extract from the leaves of Petasites japonicus Maxim (PJ) was studied for its (anti-)mutagenic effect with the SOS chromotest and reverse mutation assay. The (anti-)carcinogenic effects were evaluated by the cytotoxicity on human cancer line cells and by the function and the expression of gap junctions in rat liver epithelial cell. PJ extracts significantly decreased spontaneous beta-galactosidase activity and beta-galactosidase activity induced by a mutagen, ICR, in Salmonella (S.) typhimurium TA 1535/pSK 1002. All doses of the extract (0.08-100 mg/plate) decreased the reversion frequency induced by benzo (alpha)pyrene (BaP) in S. typhimurium TA 98. It decreased not only the spontaneous reversion frequency but also that induced by BaP in S. typhimurium TA 100. PJ extract showed greater cytotoxic effects on human stomach, colon and uterus cancer cells than on other cancer cell types and normal rat liver epithelial cells. Dye transfers though gap junctions were significantly increased by PJ extracts at concentrations greater than 200 microg/mL and the inhibition of dye transfer by 12-O-tetradecanoylphorobol-13-acetate (TPA) was obstructed in all concentrations of PJ. PJ significantly increased the numbers of gap junction protein connexin 43, and increased the protein expression decreased by TPA in a dose-dependent manner. Based on these findings, PJ is suggested to contain antimutagenic and anticarcionogenic compounds.
MeSH Terms
Figure
Reference
-
1. Bickel D, Röder T, Bestmann HJ, Brune K. Identification and characterization of inhibitors of peptido-leukotriene-synthesis from Petasites hybridus. Planta Med. 1994. 50:318–322.2. Brockman HE, Stack HF, Waters MD. Antimutagenicity profiles of some natural substances. Mutat Res. 1992. 267:157–172.
Article3. Brune K, Bickel D, Peskar BA. Gastro-protective effects by extracts of Petasites hybridus: the role of inhibition of peptido-leukotriene synthesis. Planta Med. 1993. 59:494–496.
Article4. Edenharder R, Leopold C, Kries M. Modifying actions of solvent extracts from fruit and vegetable residues on 2-amino-3-methylimidazo[4,5-f]quinoline (IQ) and 2-amino-3,4-dimethylimidazo[4,5-f]quinoxaline (MeIQx) induced mutagenesis in Salmonella typhimurium TA 98. Mutat Res. 1995. 341:303–318.
Article5. Edwards GO, Jondhale S, Chen T, Chipman JK. A quantitative inverse relationship between connexin32 expression and cell proliferation in a rat hepatoma cell line. Toxicology. 2008. 253:46–52.
Article6. El-Fouly MH, Trosko JE, Chang CC. Scrape-loading and dye transfer: A rapid and simple technique to study gap junctional intercellular communication. Exp Cell Res. 1987. 168:422–430.7. Enomoto T, Yamasaki H. Lack of intercellular communication between chemically transformed and surrounding nontransformed BALB/C 3T3 cells. Cancer Res. 1984. 44:5200–5203.8. Fitzgerald DJ, Swierenga SH, Mesnil M, Piccoli C, Marceau N, Yamasaki H. Gap junctional intercellular communication and connexin expression in normal and SV 40-transformed human liver cells in vitro. Cancer Lett. 1993. 71:157–165.
Article9. Hirono I. Natural carcinogenic products of plant origin. Crit Rev Toxicol. 1981. 8:235–377.
Article10. Hirono I, Mori H, Yamada K, Hirata Y, Haga M, Tamematsu H, Kanie S. Carcinogenic activity of petasitenine, a new pyrrolizidine alkaloid isolated from Petasites japonicus Maxim. J Natl Cancer Inst. 1977. 58:1155–1157.
Article11. Holder JW, Elmore E, Barrett JC. Gap junction function and cancer. Cancer Res. 1993. 53:3475–3485.12. Iriye R, Furukawa K, Nishida R, Kim C, Fukami H. Isolation and synthesis of a new bio-antimutagen, petasiphenol, from scapes of Petasites japonicum. Biosci Biotechnol Biochem. 1992. 56:1773–1775.
Article13. Ishibashi K, Takahashi W, Takei H, Kakinuma K. Possible interaction of thiol groups of proteins with anti-mutagens containing a conjugated carbonyl structure. Agric Biol Chem. 1987. 51:1045–1049.
Article14. Jarry H, Stromeier S, Wuttke W, Nahrstedt A. Petasiphenone, a phenol isolated from Cimicifuga racemosa, in vitro inhibits proliferation of the human prostate cancer cell line LNCaP. Planta Med. 2007. 73:184–187.
Article15. Jeong SH, Cho MH, Cho JH. Effects of cadmium on gap junctional intercellular communication in WB-F344 rat liver epithelial cells. Hum Exp Toxicol. 2001. 20:577–583.
Article16. Kakinuma K, Koike J, Ishibashi K, Takahashi W, Takei H. Structure-activity relationship and design of an antimutagen against the UV-induced mutation of Escherichia coli. Agric Biol Chem. 1986. 50:625–631.
Article17. Klaunig JE, Ruch RJ. Role of inhibition of intercellular communication in carcinogenesis. Lab Invest. 1990. 62:135–146.
Article18. Kuroda Y, Hara Y. Antimutagenic and anticarcinogenic activity of tea polyphenols. Mutat Res. 1999. 436:69–97.
Article19. Loewenstein WR. Junctional intercellular communication and the control of growth. Biochim Biophys Acta. 1979. 560:1–65.
Article20. Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000. 21:485–495.
Article21. Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983. 113:173–215.
Article22. Matesic DF, Rupp HL, Bonney WJ, Ruch RJ, Trosko JE. Changes in gap-junction permeability, phosphorylation, and number mediated by phorbol ester and non-phorbol-ester tumor promoters in rat liver epithelial cells. Mol Carcinog. 1994. 10:226–236.
Article23. Matsubara K, Mori M, Mizushina Y. Petasiphenol which inhibits DNA polymerase lambda activity is an inhibitor of in vitro angiogenesis. Oncol Rep. 2004. 11:447–451.24. Miller JH. Experiments in Molecular Genetics. 1972. New York: Cold Spring Harbor Laboratory;352–355.25. Mizushina Y, Kamisuki S, Kasai N, Ishidoh T, Shimazaki N, Takemura M, Asahara H, Linn S, Yoshida S, Koiwai O, Sugawara F, Yoshida H, Sakaguchi K. Petasiphenol: a DNA polymerase λ inhibitor. Biochemistry. 2002. 41:14463–14471.
Article26. Mori H, Ushimaru Y, Tanaka T, Hirono I. Effect of carbon tetrachloride on carcinogenicity of Petasites japonicus and transplantability of induced tumors. Gann. 1977. 68:841–845.27. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983. 65:55–63.
Article28. Oda Y, Nakamura S, Oki I, Kato T, Shinagawa H. Evaluation of the new system (umu-test) for the detection of environmental mutagens and carcinogens. Mutat Res. 1985. 147:219–229.
Article29. Oda Y, Yamazaki H, Watanabe M, Nohmi T, Shimada T. Highly sensitive umu test system for the detection of mutagenic nitroarenes in Salmonella typhimurium NM3009 having high O-acetyltransferase and nitroreductase activities. Environ Mol Mutagen. 1993. 21:357–364.
Article30. Ogawara K, Un K, Minato K, Tanaka K, Higaki K, Kimura T. Determinants for in vivo anti-tumor effects of PEG liposomal doxorubicin: importance of vascular permeability within tumors. Int J Pharm. 2008. 359:234–240.
Article31. Ong KC, Khoo HE. Biological effects of myricetin. Gen Pharmacol. 1997. 29:121–126.
Article32. Rosenkranz HS, Pollack N, Cunningham AR. Exploring the relationship between the inhibition of gap junctional intercellular communication and other biological phenomena. Carcinogenesis. 2000. 21:1007–1011.
Article33. Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003. 3:768–780.
Article34. Thomet OA, Schapowal A, Heinisch IV, Wiesmann UN, Simon HU. Anti-inflammatory activity of an extract of Petasites hybridus in allergic rhinitis. Int Immunopharmacol. 2002. 2:997–1006.
Article35. Tobinaga S, Takeuchi N, Kasama T, Yamashita J, Aida Y, Kaneko Y. Anti-histaminic and anti-allergic principles of Petasites japonicus Maxim. Chem Pharm Bull (Tokyo). 1983. 31:745–748.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Aqueous extract of Petasites japonicus leaves promotes osteoblast differentiation via up-regulation of Runx2 and Osterix in MC3T3-E1 cells
- Antimutagenic and Antioxidant Activities of Thai Rice Brans
- Antimicrobial Effect of Pulsatilla Koreana Extracts on Food-Borne Pathogens
- Quantitative Determination of Bakkenolide D in Petasites japonicus and Farfugium japonicum by HPLC/UV
- In Vitro Screening of Anti-lice Activity of Pongamia pinnata Leaves