Nutr Res Pract.
2021 Oct;15(5):579-590. 10.4162/nrp.2021.15.5.579.
Aqueous extract of Petasites japonicus leaves promotes osteoblast differentiation via up-regulation of Runx2 and Osterix in MC3T3-E1 cells
- Affiliations
-
- 1Regional Strategic Industry Innovation Center, Hallym University, Chuncheon 24252, Korea
- 2Department of Food Science & Nutrition, Dongseo University, Busan 47011, Korea
- KMID: 2520660
- DOI: http://doi.org/10.4162/nrp.2021.15.5.579
Abstract
- BACKGROUND/OBJECTIVES
Petasites japonicus Maxim (P. japonicus) has been used as an edible and medicinal plant and contains many bioactive compounds. The purpose of this study is to investigate the effect of P. japonicus on osteogenesis.
MATERIALS/METHODS
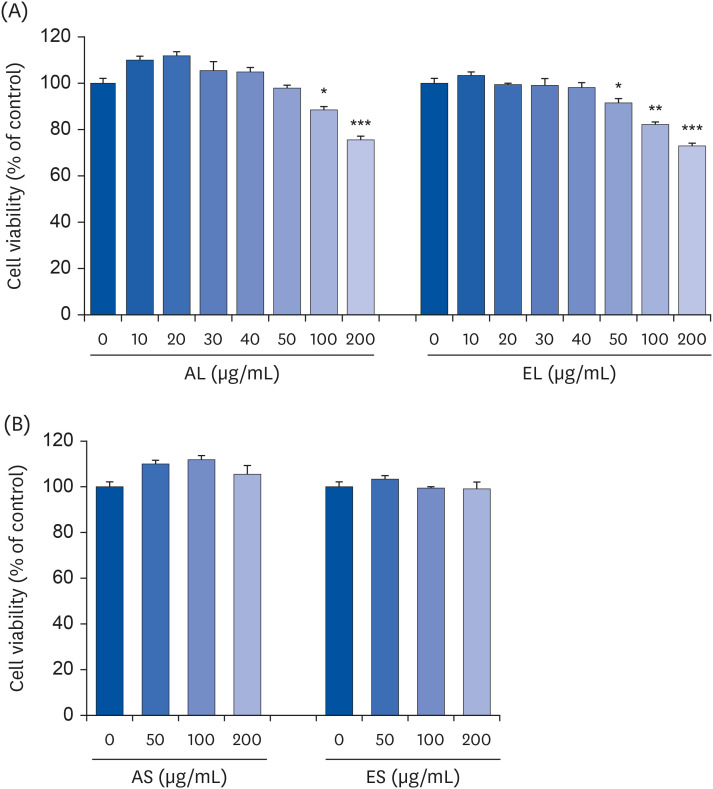
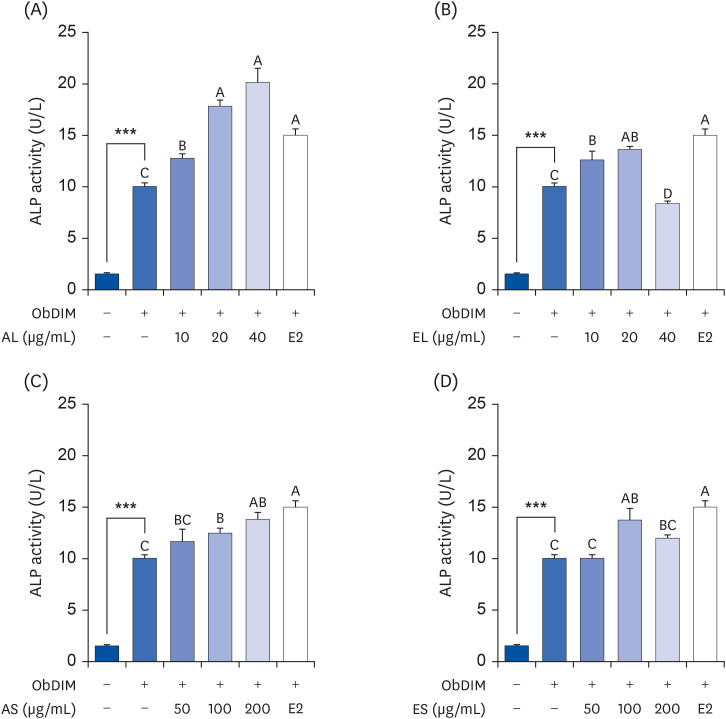
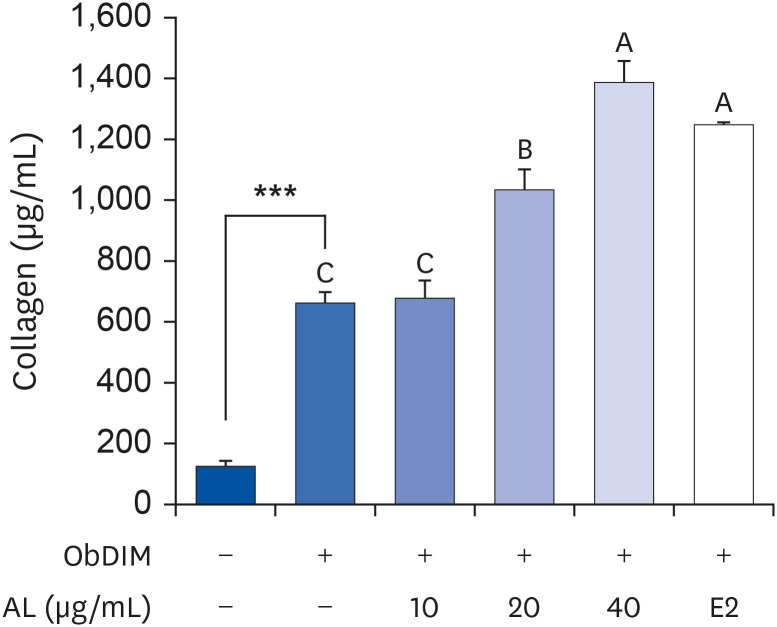
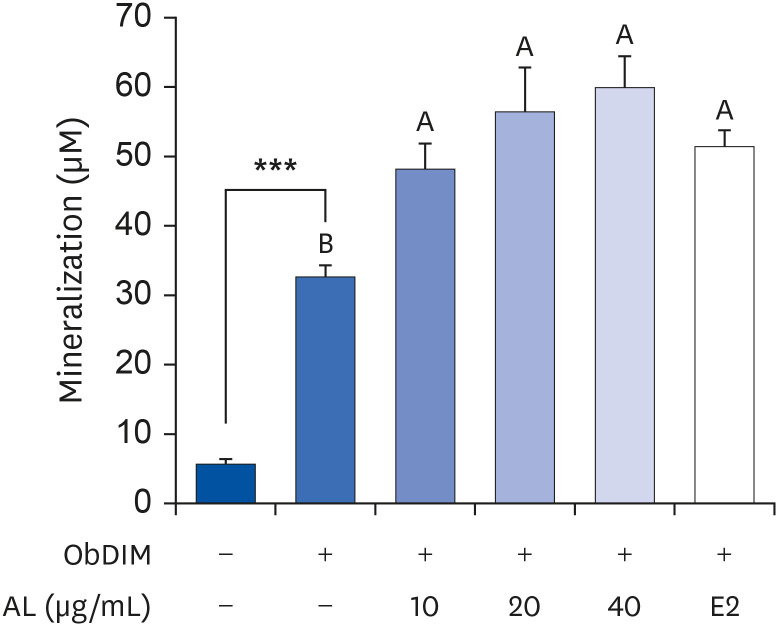
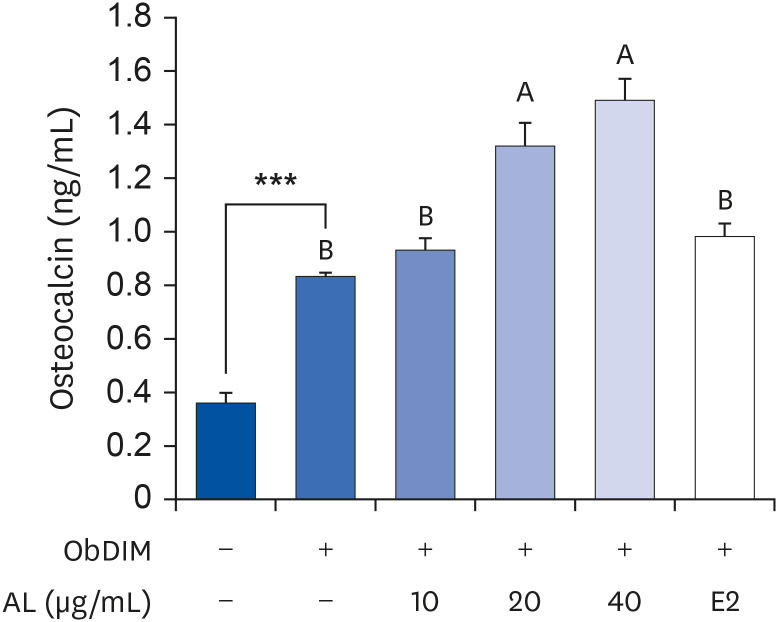
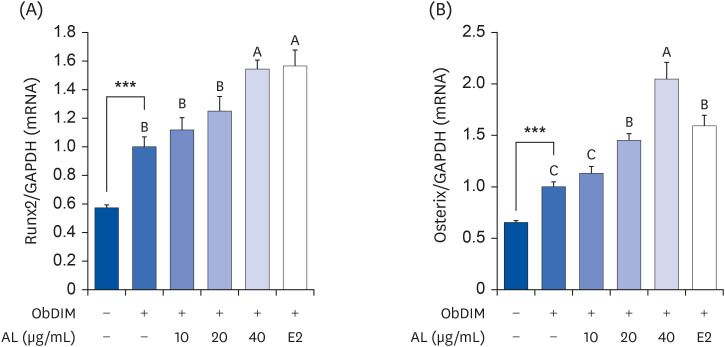
The leaves and stems of P. japonicus were separated and extracted with hot water or ethanol, respectively. The total phenolic compound and total polyphenol contents of each extract were measured, and alkaline phosphatase (ALP) activity of each extract was evaluated to determine their effect on bone metabolism. To investigate the effect on osteoblast differentiation of the aqueous extract of P. japonicus leaves (AL), which produced the highest ALP activity among the tested extracts, collagen content was measured using the Sirius Red staining method, mineralization using the Alizarin Red S staining method, and osteocalcin production through enzyme-linked immunosorbent assay analysis. Also, realtime reverse transcription polymerase chain reaction was performed to investigate the mRNA expression levels of Runt-related transcriptional factor 2 (Runx2) and Osterix.
RESULTS
Among the 4 P. japonicus extracts, AL had the highest values in all of the following measures: total phenolic compounds, total polyphenols, and ALP activity, which is a major biomarker of osteoblast differentiation. The AL-treated MC3T3-E1 cells showed significant increases in induced osteoblast differentiation, collagen synthesis, mineralization, and osteocalcin production. In addition, mRNA expressions of Runx2 and Osterix, transcription factors that regulate osteoblast differentiation, were significantly increased.
CONCLUSIONS
These results suggest that AL can regulate osteoblasts differentiation, at least in part through Runx2 and Osterix. Therefore, it is highly likely that P. japonicus will be useful as an alternate therapeutic for the prevention and treatment of osteoporosis.
Keyword
Figure
Reference
-
1. Wong SK, Chin KY, Ima-Nirwana S. The osteoprotective effects of kaempferol: the evidence from in vivo and in vitro studies. Drug Des Devel Ther. 2019; 13:3497–3514.2. He JB, Chen MH, Lin DK. New insights into the tonifying kidney-yin herbs and formulas for the treatment of osteoporosis. Arch Osteoporos. 2017; 12:14. PMID: 28127706.
Article3. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J; Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002; 288:321–333. PMID: 12117397.4. O'Ryan FS, Lo JC. Bisphosphonate-related osteonecrosis of the jaw in patients with oral bisphosphonate exposure: clinical course and outcomes. J Oral Maxillofac Surg. 2012; 70:1844–1853. PMID: 22595135.5. Wang S, Jin DQ, Xie C, Wang H, Wang M, Xu J, Guo Y. Isolation, characterization, and neuroprotective activities of sesquiterpenes from Petasites japonicus . Food Chem. 2013; 141:2075–2082. PMID: 23870930.6. Sun ZL, Gao GL, Luo JY, Zhang XL, Zhang M, Feng J. A new neuroprotective bakkenolide from the rhizome of Peatasites tatewakianus . Fitoterapia. 2011; 82:410–414.7. Hiemori-Kondo M. Antioxidant compounds of Petasites japonicus and their preventive effects in chronic diseases: a review. J Clin Biochem Nutr. 2020; 67:10–18. PMID: 32801463.8. Ahn EM, Asamenew G, Kim HW, Lee SH, Yoo SM, Cho SM, Cha YS, Kang MS. Anti-obesity effects of Petasites japonicus (Meowi) ethanol extract on RAW 264.7 macrophages and 3T3-L1 adipocytes and its characterization of polyphenolic compounds. Nutrients. 2020; 12:1261.9. Kang HG, Jeong SH, Cho JH. Antimutagenic and anticarcinogenic effect of methanol extracts of Petasites japonicus Maxim leaves. J Vet Sci. 2010; 11:51–58. PMID: 20195065.10. Kim HJ, Park SY, Lee HM, Seo DI, Kim YM. Antiproliferative effect of the methanol extract from the roots of Petasites japonicus on Hep3B hepatocellular carcinoma cells in vitro and in vivo . Exp Ther Med. 2015; 9:1791–1796. PMID: 26136894.11. Kim KM, Im AR, Lee S, Chae S. Dual protective effects of flavonoids from Petasites japonicus against UVB-induced apoptosis mediated via HSF-1 activated heat shock proteins and Nrf2-activated heme oxygenase-1 pathways. Biol Pharm Bull. 2017; 40:765–773. PMID: 28566621.12. Kim N, Choi JG, Park S, Lee JK, Oh MS. Butterbur leaves attenuate memory impairment and neuronal cell damage in amyloid beta-induced Alzheimer's disease models. Int J Mol Sci. 2018; 19:1644.
Article13. Matsumoto T, Imahori D, Saito Y, Zhang W, Ohta T, Yoshida T, Nakayama Y, Ashihara E, Watanabe T. Cytotoxic activities of sesquiterpenoids from the aerial parts of Petasites japonicus against cancer stem cells. J Nat Med. 2020; 74:689–701. PMID: 32535872.14. Shimoda H, Tanaka J, Yamada E, Morikawa T, Kasajima N, Yoshikawa M. Anti type I allergic property of Japanese butterbur extract and its mast cell degranulation inhibitory ingredients. J Agric Food Chem. 2006; 54:2915–2920. PMID: 16608208.
Article15. Lee KP, Kang S, Park SJ, Choi YW, Lee YG, Im DS. Anti-allergic and anti-inflammatory effects of bakkenolide B isolated from Petasites japonicus leaves. J Ethnopharmacol. 2013; 148:890–894. PMID: 23711828.16. Choi JY, Desta KT, Saralamma VVG, Lee SJ, Lee SJ, Kim SM, Paramanantham A, Lee HJ, Kim YH, Shin HC, Shim JH, Warda M, Hacımüftüoğlu A, Jeong JH, Shin SC, Kim GS, Abd El-Aty AM. LC-MS/MS characterization, anti-inflammatory effects and antioxidant activities of polyphenols from different tissues of Korean Petasites japonicus (Meowi). Biomed Chromatogr. 2017; 31:e4033.17. Lee JS, Jeong M, Park S, Ryu SM, Lee J, Song Z, Guo Y, Choi JH, Lee D, Jang DS. Chemical constituents of the leaves of butterbur (Petasites japonicus) and their anti-inflammatory effects. Biomolecules. 2019; 9:806.18. Guo L, Li K, Cui ZW, Kang JS, Son BG, Choi YW. S-Petasin isolated from Petasites japonicus exerts anti-adipogenic activity in the 3T3-L1 cell line by inhibiting PPAR-γ pathway signaling. Food Funct. 2019; 10:4396–4406. PMID: 31282906.19. Lee JS, Yang EJ, Yun CY, Kim DH, Kim IS. Suppressive effect of Petasites japonicus extract on ovalbumin-induced airway inflammation in an asthmatic mouse model. J Ethnopharmacol. 2011; 133:551–557. PMID: 21029770.20. Singleton VL, Rossi TA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965; 16:144–158.21. Moreno MI, Isla MI, Sampietro AR, Vattuone MA. Comparison of the free radical-scavenging activity of propolis from several regions of Argentina. J Ethnopharmacol. 2000; 71:109–114. PMID: 10904153.
Article22. Kim EJ, Holthuizen PE, Park HS, Ha YL, Jung KC, Park JH. Trans-10,cis-12-conjugated linoleic acid inhibits Caco-2 colon cancer cell growth. Am J Physiol Gastrointest Liver Physiol. 2002; 283:G357–67. PMID: 12121883.
Article23. Sakamura S, Yoshihara T, Toyoda K. The constituents of Petasites japonicas: structures of fukiic acid and fukinolic acid. Agric Biol Chem. 1973; 37:1915–1921.24. Calderón-Montaño JM, Burgos-Morón E, Pérez-Guerrero C, López-Lázaro M. A review on the dietary flavonoid kaempferol. Mini Rev Med Chem. 2011; 11:298–344. PMID: 21428901.25. Imran M, Rauf A, Shah ZA, Saeed F, Imran A, Arshad MU, Ahmad B, Bawazeer S, Atif M, Peters DG, Mubarak MS. Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: a comprehensive review. Phytother Res. 2019; 33:263–275. PMID: 30402931.
Article26. Heino TJ, Hentunen TA. Differentiation of osteoblasts and osteocytes from mesenchymal stem cells. Curr Stem Cell Res Ther. 2008; 3:131–145. PMID: 18473879.
Article27. Vimalraj S, Arumugam B, Miranda PJ, Selvamurugan N. Runx2: Structure, function, and phosphorylation in osteoblast differentiation. Int J Biol Macromol. 2015; 78:202–208. PMID: 25881954.
Article28. Komori T. Signaling networks in RUNX2-dependent bone development. J Cell Biochem. 2011; 112:750–755. PMID: 21328448.
Article29. Kawane T, Qin X, Jiang Q, Miyazaki T, Komori H, Yoshida CA, Matsuura-Kawata VK, Sakane C, Matsuo Y, Nagai K, Maeno T, Date Y, Nishimura R, Komori T. Runx2 is required for the proliferation of osteoblast progenitors and induces proliferation by regulating Fgfr2 and Fgfr3. Sci Rep. 2018; 8:13551. PMID: 30202094.
Article30. Bruderer M, Richards RG, Alini M, Stoddart MJ. Role and regulation of RUNX2 in osteogenesis. Eur Cell Mater. 2014; 28:269–286. PMID: 25340806.
Article31. Fu H, Doll B, McNelis T, Hollinger JO. Osteoblast differentiation in vitro and in vivo promoted by Osterix. J Biomed Mater Res A. 2007; 83:770–778. PMID: 17559111.32. Zhang C. Transcriptional regulation of bone formation by the osteoblast-specific transcription factor Osx. J Orthop Surg. 2010; 5:37.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Zinc modulation of osterix in MC3T3-E1 cells
- Zinc upregulates bone-specific transcription factor Runx2 expression via BMP-2 signaling and Smad-1 phosphorylation in osteoblasts
- Secreotory Leukocyte Protease Inhibitor Regulates Bone Formation via RANKL, OPG, and Runx2 in Rat Periodontitis and MC3T3-E1 Preosteoblast
- Effect of Extracts from Safflower Seeds on Osteoblastic Differentiation and Intracellular Free Calcium Concentration in MC3T3-E1 Cells
- Remifentanil promotes osteoblastogenesis by upregulating Runx2/osterix expression in preosteoblastic C2C12 cells