J Vet Sci.
2007 Mar;8(1):27-38. 10.4142/jvs.2007.8.1.27.
Estrogen receptor independent neurotoxic mechanism of bisphenol A, an environmental estrogen
- Affiliations
-
- 1College of Pharmacy and CBITRC, Chungbuk National University, Cheongju 361-763, Korea. jinthong@chungbuk.ac.kr
- 2College of Veterinary Medicine and Research Institute of Veterinary Medicine, Chungbuk National University, Cheongju 361-763, Korea.
- 3National Institute of Toxicological Research, Korea Food and Drug Administration, Seoul 122-704, Korea.
- KMID: 1104233
- DOI: http://doi.org/10.4142/jvs.2007.8.1.27
Abstract
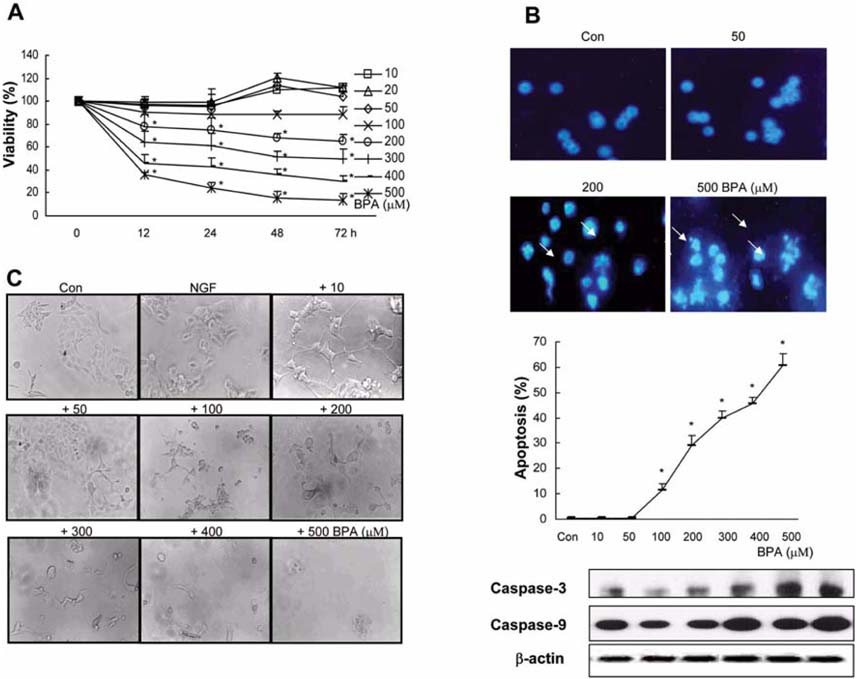
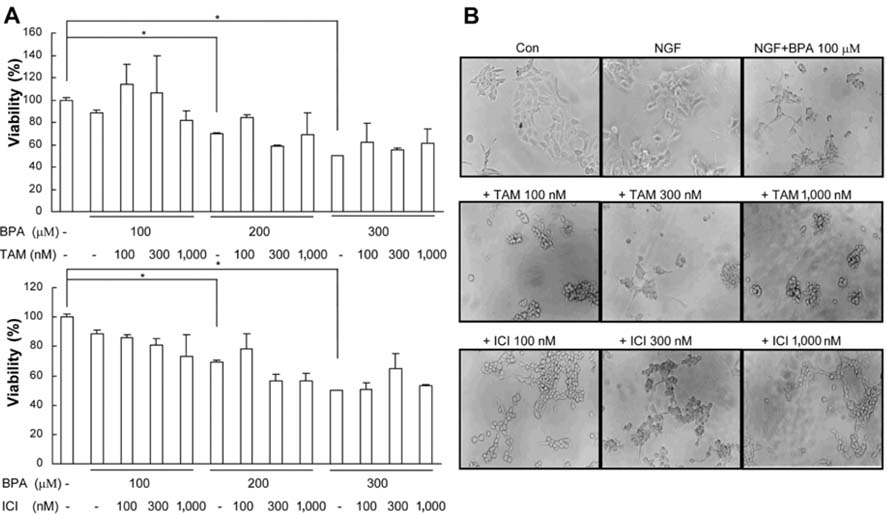
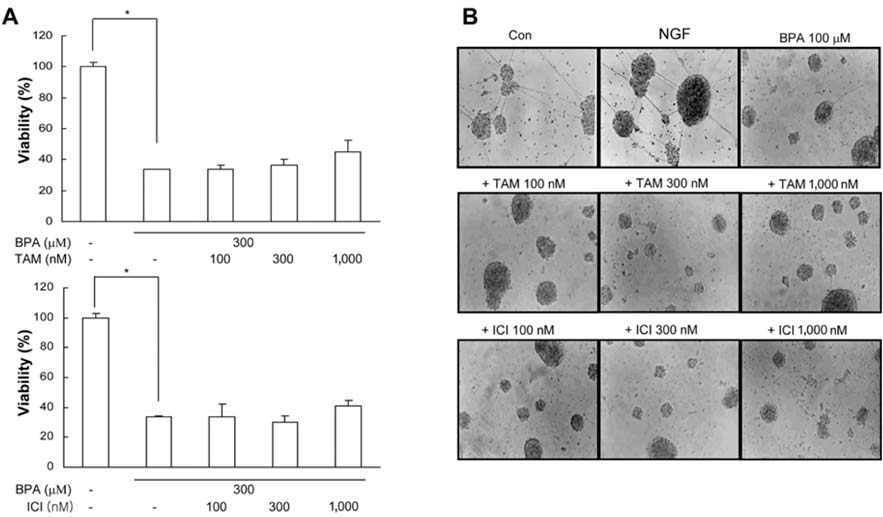
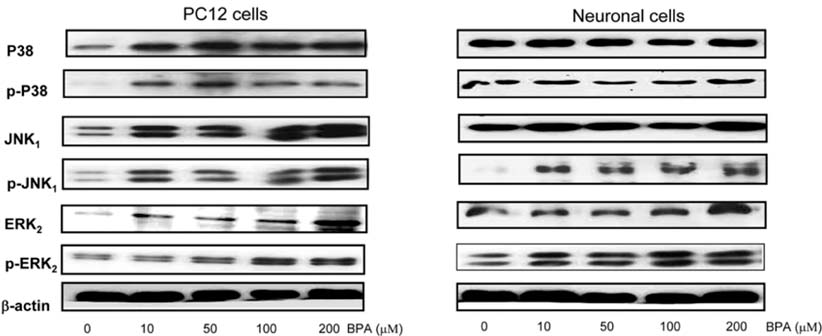
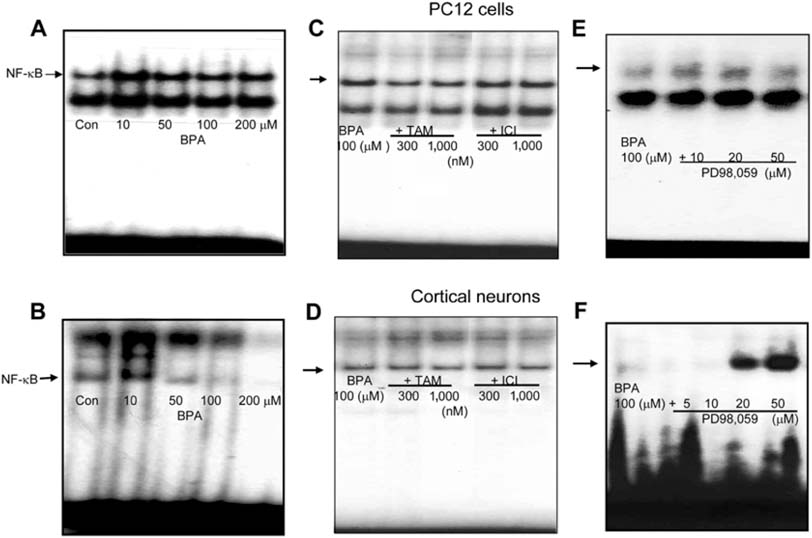
- Bisphenol A (BPA), a ubiquitous environmental contaminant, has been shown to cause developmental toxicity and carcinogenic effects. BPA may have physiological activity through estrogen receptor (ER) -alpha and -beta, which are expressed in the central nervous system. We previously found that exposure of BPA to immature mice resulted in behavioral alternation, suggesting that overexposure of BPA could be neurotoxic. In this study, we further investigated the molecular neurotoxic mechanisms of BPA. BPA increased vulnerability (decrease of cell viability and differentiation, and increase of apoptotic cell death) of undifferentiated PC12 cells and cortical neuronal cells isolated from gestation 18 day rat embryos in a concentration-dependent manner (more than 50 micrometer). The ER antagonists, ICI 182,780, and tamoxifen, did not block these effects. The cell vulnerability against BPA was not significantly different in the PC12 cells overexpressing ER-alpha and ER-beta compared with PC12 cells expressing vector alone. In addition, there was no difference observed between BPA and 17-beta estradiol, a well-known agonist of ER receptor in the induction of neurotoxic responses. Further study of the mechanism showed that BPA significantly activated extracellular signal-regulated kinase (ERK) but inhibited anti-apoptotic nuclear factor kappa B (NF-kappaB) activation. In addition, ERK-specific inhibitor, PD 98,059, reversed BPA-induced cell death and restored NF-kappaB activity. This study demonstrated that exposure to BPA can cause neuronal cell death which may eventually be related with behavioral alternation in vivo. However, this neurotoxic effect may not be directly mediated through an ER receptor, as an ERK/NF-kappaB pathway may be more closely involved in BPA-induced neuronal toxicity.
MeSH Terms
-
Animals
Apoptosis/drug effects
Blotting, Western
Cell Differentiation/drug effects
Cell Survival/drug effects
Estradiol/analogs & derivatives/pharmacology
Estrogens, Non-Steroidal/*toxicity
Flavonoids/pharmacology
NF-kappa B/metabolism
Neurons/*drug effects/physiology
PC12 Cells
Phenols/*toxicity
Rats
Receptors, Estrogen/metabolism
Tamoxifen/pharmacology
Figure
Reference
-
1. Alessandrini A, Namura S, Moskowitz MA, Bonventre JV. MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc Natl Acad Sci USA. 1999. 96:12866–12869.
Article2. Aloisi AM, Della Seta D, Rendo C, Ceccarelli I, Scaramuzzino A, Farabollini F. Exposure to the estrogenic pollutant bisphenol A affects pain behavior induced by subcutaneous formalin injection in male and female rats. Brain Res. 2002. 937:1–7.
Article3. Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S. Differential response of estrogen receptor and estrogen receptor to partial estrogen agonist/ antagonists. Mol Pharmacol. 1998. 54:105–112.
Article4. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976. 72:248–254.
Article5. Brotons JA, Olea-Serrano MF, Villalobos M, Pedraza V, Olea N. Xenoestrogens released from lacquer coating in food cans. Environ Health Perspect. 1995. 103:608–612.6. Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits atp-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003. 118:941–948.
Article7. Coleman KM, Dutertre M, El-Gharbawy A, Rowan BG, Weigel NL, Smith CL. Mechanistic differences in the activation of estrogen receptor-α (ERα)-and ERβ-dependent gene expression by cAMP signaling pathway(s). J Biol Chem. 2003. 278:12834–12845.
Article8. Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999. 20:358–417.
Article9. Foehr ED, Bohuslav J, Chen LF, DeNoronha C, Geleziunas R, Lin X, O'Mahony A, Greene WC. The NF-κB-inducing kinase induces PC12 cell differentiation and prevents apoptosis. J Biol Chem. 2000. 275:34021–34024.
Article10. Gomez-Santos C, Ferrer I, Reiriz J, Vinals F, Barrachina M, Ambrosio S. MPP+ increases α-synuclein expression and ERK/MAP-kinase phosphorylation in human neuroblastoma SH-SY5Y cells. Brain Res. 2002. 935:32–39.11. Guillette LJ Jr, Gross TS, Gross DA, Rooney AA, Percival HF. Gonadal steroidogenesis in vitro from juvenile alligators obtained from contaminated or control lakes. Environ Health Perspect. 1995. 103:Suppl. 31–36.
Article12. Gursoy E, Cardounel A, Kalimi M. The environmental estrogenic compound bisphenol A exerts estrogenic effects on mouse hippocampal (HT-22) cells: neuroprotection against glutamate and amyloid beta protein toxicity. Neurochem Int. 2001. 38:181–186.
Article13. Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-β immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001. 142:3261–3264.
Article14. Iida H, Maehara K, Doiguchi M, Mori T, Yamada F. Bisphenol A-induced apoptosis of cultured rat Sertoli cells. Reprod Toxicol. 2003. 17:457–464.
Article15. Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002. 17:2839–2841.
Article16. Jiang B, Xu S, Hou X, Pimentel DR, Brecher P, Cohen RA. Temporal control of NF-κB activation by ERK differentially regulates interleukin-1β-induced gene expression. J Biol Chem. 2004. 279:1323–1329.
Article17. Jung KM, Park KS, Oh JH, Jung SY, Yang KH, Song YS, Son DJ, Park YH, Yun YP, Lee MK, Oh KW, Hong JT. Activation of p38 mitogen-activated protein kinase and activator protein-1 during the promotion of neurite extension of PC-12 cells by 15-deoxy-Δ12,14-prostaglandin J2. Mol Pharmacol. 2003. 63:607–616.
Article18. Kim JC, Shin HC, Cha SW, Koh WS, Chung MK, Han SS. Evaluation of developmental toxicity in rats exposed to the environmental estrogen bisphenol A during pregnancy. Life Sci. 2001. 69:2611–2625.
Article19. Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JÅ. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997. 138:863–870.
Article20. Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JÅ. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998. 139:4252–4263.
Article21. Küppers E, Ivanova T, Karolczak M, Lazarov N, Föhr K, Beyer C. Classical and nonclassical estrogen action in the developing midbrain. Horm Behav. 2001. 40:196–202.
Article22. Lee YM, Lee SM, Son DJ, Lee SY, Park HJ, Nam SY, Kim DJ, Yun YW, Yoo HS, Oh KW, Kim TS, Han YS. Bisphenol a disturbs intracellular calcium homeostasis and its relationship with cytotoxicity. J Toxicol Public Health. 2004. 20:57–66.23. Linford NJ, Yang Y, Cook DG, Dorsa DM. Neuronal apoptosis resulting from high doses of the isoflavone genistein: role for calcium and p42/44 mitogen-activated protein kinase. J Pharmacol Exp Ther. 2001. 299:67–75.24. Moore SA, Huang N, Hinthong O, Andres RD, Grammatopoulos TN, Weyhenmeyer JA. Human angiotensin II type-2 receptor inhibition of insulin-mediated ERK-2 activity via a G-protein coupled signaling pathway. Brain Res Mol Brain Res. 2004. 124:62–69.
Article25. Murasawa S, Mori Y, Nozawa Y, Masaki H, Maruyama K, Tsutsumi Y, Moriguchi Y, Shibasaki Y, Tanaka Y, Iwasaka T, Inada M, Matsubara H. Role of calcium-sensitive tyrosine kinase Pyk2/CAKβ/RAFTK in angiotensin II induced Ras/ERK signaling. Hypertension. 1998. 32:668–675.
Article26. Nilsson S, Mäkelä S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JÅ. Mechanisms of estrogen action. Physiol Rev. 2001. 81:1535–1565.
Article27. Oh JH, Jung HK, Park YJ, Kim CK, Chung SY, Park NG, Yun YW, Kim DJ, Ha TY, Song YS, Lee YM, Oh K, Hong JT. Inhibitory effects of ochratoxin A on nerve growth factor-induced neurite extension through downregulation of p38 MAP kinase and AP-1 activation in cultured pheochromocytoma cells. J Toxicol Environ Health A. 2004. 67:357–371.
Article28. Oka T, Adati N, Shinkai T, Sakuma K, Nishimura T, Kurose K. Bisphenol A induces apoptosis in central neural cells during early development of Xenopus laevis. Biochem Biophys Res Commun. 2003. 312:877–882.
Article29. O'Neill LAJ, Kaltschmidt C. NF-κB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997. 20:252–258.30. Papaconstantinou AD, Umbreit TH, Fisher BR, Goering PL, Lappas NT, Brown KM. Bisphenol A-induced increase in uterine weight and alterations in uterine morphology in ovariectomized B6C3F1 mice: role of the estrogen receptor. Toxicol Sci. 2000. 56:332–329.
Article31. Paris F, Balaguer P, Térouanne B, Servant N, Lacoste C, Cravedi JP, Nicolas JC, Sultan C. Phenylphenols, biphenols, bisphenol-A and 4-tert-octylphenol exhibit α and β estrogen activities and antiandrogen activity in reporter cell lines. Mol Cell Endocrinol. 2002. 193:43–49.
Article32. Purves T, Middlemas A, Agthong S, Jude EB, Boulton AJ, Fernyhough P, Tomlinson DR. A role for mitogen-activated protein kinases in the etiology of diabetic neuropathy. FASEB J. 2001. 15:2508–2514.
Article33. Raychoudhury SS, Flowers AF, Millette CF, Finlay MF. Toxic effects of polychlorinated biphenyls on cultured rat Sertoli cells. J Androl. 2000. 21:964–973.34. Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, Kojima Y, Piao JH, Yagita H, Okumura K, Doi T, Nakano H. NF-κB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003. 22:3898–3909.
Article35. Seo SR, Chong SA, Lee SI, Sung JY, Ahn YS, Chung KC, Seo JT. Zn2+-induced ERK activation mediated by reactive oxygen species causes cell death in differentiated PC12 cells. J Neurochem. 2001. 78:600–610.
Article36. Seoung MJ, Shin IC, Lee YM, Son DJ, Song YS, Jeon KH, Kim YB, Lee BJ, Kim DJ, Yun YW, Kim TS, Han SY. Behavior alterations and expression of estrogen receptors in mice exposed to Bisphenol A. J Toxicol Public Health. 2004. 20:67–77.37. Shim GJ, Wang L, Andersson S, Nagy N, Kis LL, Zhang Q, Mäkelä S, Warner M, Gustafsson JÅ. Disruption of the estrogen receptor β gene in mice causes myeloproliferative disease resembling chronic myeloid leukemia with lymphoid blast crisis. Proc Natl Acad Sci USA. 2003. 100:6694–6699.
Article38. Sogawa N, Onodera K, Sogawa CA, Mukubo Y, Fukuoka H, Oda N, Furuta H. Bisphenol A enhances cadmium toxicity through estrogen receptor. Methods Find Exp Clin Pharmacol. 2001. 23:395–399.
Article39. Sugita-Konishi Y, Shimura S, Nishikawa T, Sunaga F, Naito H, Suzuki Y. Effect of Bisphenol A on non-specific immunodefenses against non-pathogenic Escherichia coli. Toxicol Lett. 2003. 136:217–227.
Article40. Takai Y, Tsutsumi O, Ikezuki Y, Hiroi H, Osuga Y, Momoeda M, Yano T, Taketani Y. Estrogen receptor-mediated effects of a xenoestrogen, bisphenol A, on preimplantation mouse embryos. Biochem Biophys Res Commun. 2000. 270:918–921.
Article41. Takao T, Nanamiya W, Nagano I, Asaba K, Kawabata K, Hashimoto K. Exposure with the environmental estrogen bisphenol A disrupts the male reproductive tract in young mice. Life Sci. 1999. 65:2351–2357.
Article42. Takayanagi S, Tokunaga T, Liu X, Okada H, Matsushima A, Shimohigashi Y. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor γ (ERRγ) with high constitutive activity. Toxicol Lett. 2006. 167:95–105.
Article43. Washington W, Hubert L, Jones D, Gray WG. Bisphenol a binds to the low-affinity estrogen binding site. In Vitr Mol Toxicol. 2001. 14:43–51.
Article44. Zoubina EV, Smith PG. Expression of estrogen receptors alpha and beta by sympathetic ganglion neurons projecting to the proximal urethra of female rats. J Urol. 2003. 169:382–385.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Additive Estrogenic Activities of the Binary Mixtures of Four Estrogenic Chemicals in Recombinant Yeast Expressing Human Estrogen Receptor
- Effects of estrogen receptor and estrogen on the chromatin structure in estrogen receptor stable transfectants
- Understanding the Mechanistic Link between Bisphenol A and Cancer Stem Cells: A Cancer Prevention Perspective
- An Immunocytochemical Study Of Estrogen Receptor In The Prostate
- The Effect of Tamoxifen of the Estrogen Receptor cDNA-Iipofected MDA-MB-231 Human Breast Cancer Cells