J Vet Sci.
2009 Mar;10(1):23-28. 10.4142/jvs.2009.10.1.23.
Synergistic effect of ERK inhibition on tetrandrine-induced apoptosis in A549 human lung carcinoma cells
- Affiliations
-
- 1Laboratory of Toxicology, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. mchotox@snu.ac.kr
- 2Nano Systems Institute-National Core Research Center, Seoul National University, Seoul 151-742, Korea.
- KMID: 1089342
- DOI: http://doi.org/10.4142/jvs.2009.10.1.23
Abstract
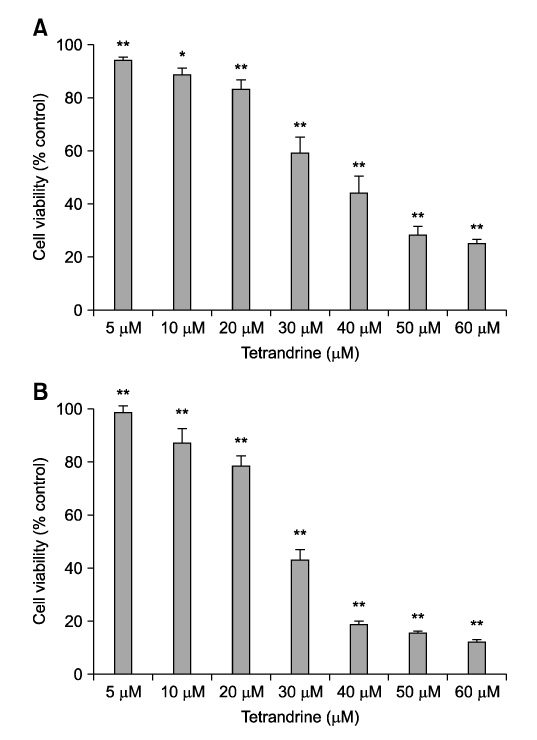
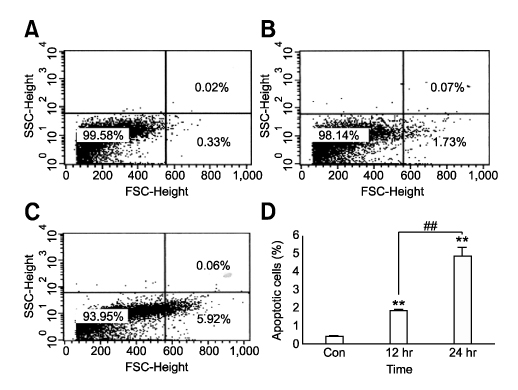
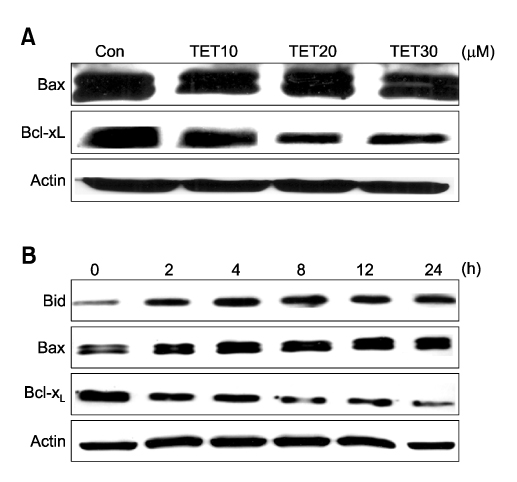
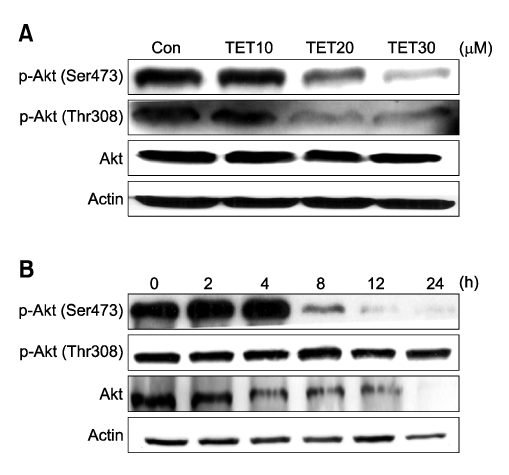
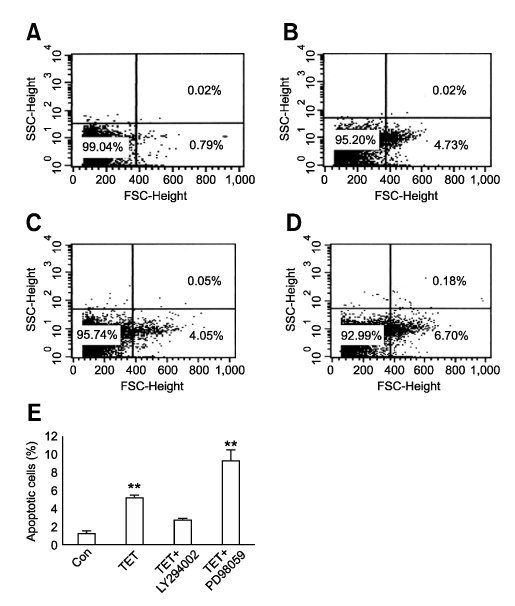
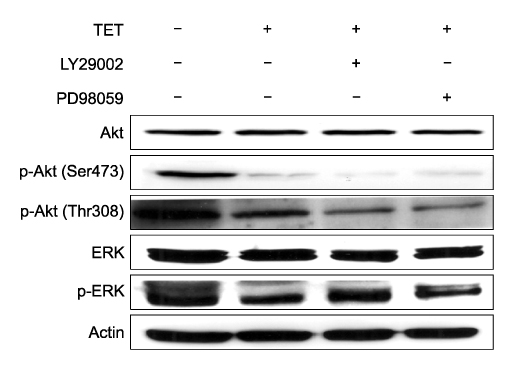
- Tetrandrine (TET), a bis-benzylisoquinoline alkaloid from the root of Stephania tetrandra, is known to have anti-tumor activity in various malignant neoplasms. However, the precise mechanism by which TET inhibits tumor cell growth remains to be elucidated. The present studies were performed to characterize the potential effects of TET on phosphoinositide 3-kinase/Akt and extracellular signal-regulated kinase (ERK) pathways since these signaling pathways are known to be responsible for cell growth and survival. TET suppressed cell proliferation and induced apoptosis in A549 human lung carcinoma cells. TET treatment resulted in a down-regulation of Akt and ERK phosphorylation in both time-/concentration-dependent manners. The inhibition of ERK using PD98059 synergistically enhanced the TET-induced apoptosis of A549 cells whereas the inhibition of Akt using LY294002 had a less significant effect. Taken together, our results suggest that TET: i) selectively inhibits the proliferation of lung cancer cells by blocking Akt activation and ii) increases apoptosis by inhibiting ERK. The treatment of lung cancers with TET may enhance the efficacy of chemotherapy and radiotherapy and increase the apoptotic potential of lung cancer cells.
Keyword
MeSH Terms
Figure
Reference
-
1. Adeyinka A, Nui Y, Cherlet T, Snell L, Watson PH, Murphy LC. Activated mitogen-activated protein kinase expression during human breast tumorigenesis and breast cancer progression. Clin Cancer Res. 2002. 8:1747–1753.2. Albanell J, Codony-Servat J, Rojo F, Del Campo JM, Sauleda S, Anido J, Raspall G, Giralt J, Roselló J, Nicholson RI, Mendelsohn J, Baselga J. Activated extracellular signal-regulated kinases: association with epidermal growth factor receptor/transforming growth factor alpha expression in head and neck squamous carcinoma and inhibition by anti-epidermal growth factor receptor treatments. Cancer Res. 2001. 61:6500–6510.3. Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996. 15:6541–6551.
Article4. Blackhall FH, Pintilie M, Michael M, Leighl N, Feld R, Tsao MS, Shepherd FA. Expression and prognostic significance of kit, protein kinase B, and mitogen-activated protein kinase in patients with small cell lung cancer. Clin Cancer Res. 2003. 9:2241–2247.5. Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999. 286:1358–1362.
Article6. Chun KH, Kosmeder JW 2nd, Sun S, Pezzuto JM, Lotan R, Hong WK, Lee HY. Effects of deguelin on the phosphatidylinositol 3-kinase/Akt pathway and apoptosis in premalignant human bronchial epithelial cells. J Natl Cancer Inst. 2003. 95:291–302.
Article7. Fry MJ. Phosphoinositide 3-kinase signalling in breast cancer: how big a role might it play? Breast Cancer Res. 2001. 3:304–312.
Article8. Hoshino R, Chatani Y, Yamori T, Tsuruo T, Oka H, Yoshida O, Shimada Y, Ari-i S, Wada H, Fujimoto J, Kohno M. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene. 1999. 18:813–822.
Article9. Hövelmann S, Beckers TL, Schmidt M. Molecular alterations in apoptotic pathways after PKB/Akt-mediated chemoresistance in NCI H460 cells. Br J Cancer. 2004. 90:2370–2377.
Article10. Hutchinson J, Jin J, Cardiff RD, Woodgett JR, Muller WJ. Activation of AKT (protein kinase B) in mammary epithelium provides a critical cell survival signal required for tumor progression. Mol Cell Biol. 2001. 21:2203–2212.
Article11. Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer Statistics, 2004. CA Cancer J Clin. 2004. 54:8–29.
Article12. Kim HW, Park IK, Cho CS, Lee KH, Beck GR Jr, Colburn NH, Cho MH. Aerosol delivery of glucosylated polyethylenimine/phosphatase and tensin homologue deleted on chromosome 10 complex suppresses Akt downstream pathways in the lung of K-ras null mice. Cancer Res. 2004. 64:7971–7976.
Article13. Lee HY. Molecular mechanisms of deguelin-induced apoptosis in transformed human bronchial epithelial cells. Biochem Pharmacol. 2004. 68:1119–1124.
Article14. Lee JH, Kang GH, Kim KC, Kim KM, Park DI, Choi BT, Kang HS, Lee YT, Choi YH. Tetrandrine-induced cell cycle arrest and apoptosis in A549 human lung carcinoma cells. Int J Oncol. 2002. 21:1239–1244.
Article15. Li B, Desai SA, MacCorkle-Chosnek RA, Fan L, Spencer DM. A novel conditional Akt 'survival switch' reversibly protects cells from apoptosis. Gene Ther. 2002. 9:233–244.
Article16. Lin X, Böhle AS, Dohrmann P, Leuschner I, Schulz A, Kremer B, Fändrich F. Overexpression of phosphatidylinositol 3-kinase in human lung cancer. Langenbecks Arch Surg. 2001. 386:293–301.
Article17. Oka H, Chatani Y, Hoshino R, Ogawa O, Kakehi Y, Terachi T, Okada Y, Kawaichi M, Kohno M, Yoshida O. Constitutive activation of mitogen-activated protein (MAP) kinases in human renal cell carcinoma. Cancer Res. 1995. 55:4182–4187.18. Pang L, Hoult JR. Cytotoxicity to macrophages of tetrandrine, an antisilicosis alkaloid, accompanied by an overproduction of prostaglandins. Biochem Pharmacol. 1997. 53:773–782.
Article19. Perkinton MS, Ip JK, Wood GL, Crossthwaite AJ, Williams RJ. Phosphatidylinositol 3-kinase is a central mediator of NMDA receptor signalling to MAP kinase (Erk1/2), Akt/PKB and CREB in striatal neurones. J Neurochem. 2002. 80:239–254.
Article20. Sebolt-Leopold JS, Dudley DT, Herrera R, Van Becelaere K, Wiland A, Gowan RC, Tecle H, Barrett SD, Bridges A, Przybranowski S, Leopold WR, Saltiel AR. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat Med. 1999. 5:810–816.
Article21. Seidman R, Gitelman I, Sagi O, Horwitz SB, Wolfson M. The role of ERK 1/2 and p38 MAP-kinase pathways in taxol-induced apoptosis in human ovarian carcinoma cells. Exp Cell Res. 2001. 268:84–92.
Article22. Sen S, D'Incalci M. Apoptosis. Biochemical events and relevance to cancer chemotherapy. FEBS Lett. 1992. 307:122–127.
Article23. Torii S, Nakayama K, Yamamoto T, Nishida E. Regulatory mechanisms and function of ERK MAP kinases. J Biochem. 2004. 136:557–561.
Article24. West KA, Brognard J, Clark AS, Linnoila IR, Yang X, Swain SM, Harris C, Belinsky S, Dennis PA. Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest. 2003. 111:81–90.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tetrandrine Exerts a Radiosensitization Effect on Human Glioma through Inhibiting Proliferation by Attenuating ERK Phosphorylation
- The Mechanism of Proteasome Inhibitor-Induced Apoptosis in Lung Cancer Cells
- Synergistic Effect of Sulindac and Simvastatin on Apoptosis in Lung Cancer A549 Cells through AKT-Dependent Downregulation of Survivin
- Antiproliferative effect of Citrus junos extracts on A549 human non-smallcell lung cancer cells
- Triptolide sensitizes lung cancer cells to TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by inhibition of NF-kappa B activation