Cancer Res Treat.
2015 Jan;47(1):90-100. 10.4143/crt.2013.194.
Synergistic Effect of Sulindac and Simvastatin on Apoptosis in Lung Cancer A549 Cells through AKT-Dependent Downregulation of Survivin
- Affiliations
-
- 1Department of Internal Medicine, Institute of Wonkwang Medical Science, Wonkwang University School of Medicine, Iksan, Korea. kshryj@wonkwang.ac.kr
- KMID: 2380394
- DOI: http://doi.org/10.4143/crt.2013.194
Abstract
- PURPOSE
Non-steroidal anti-inflammatory drugs (NSAIDs) and statins are potential chemopreventive or chemotherapeutic agents. The mechanism underlying the deregulation of survivin by NSAIDs and statins in human non-small cell lung cancer cells has not been elucidated. In this study, we investigated the synergistic interaction of sulindac and simvastatin in lung cancer A549 cells.
MATERIALS AND METHODS
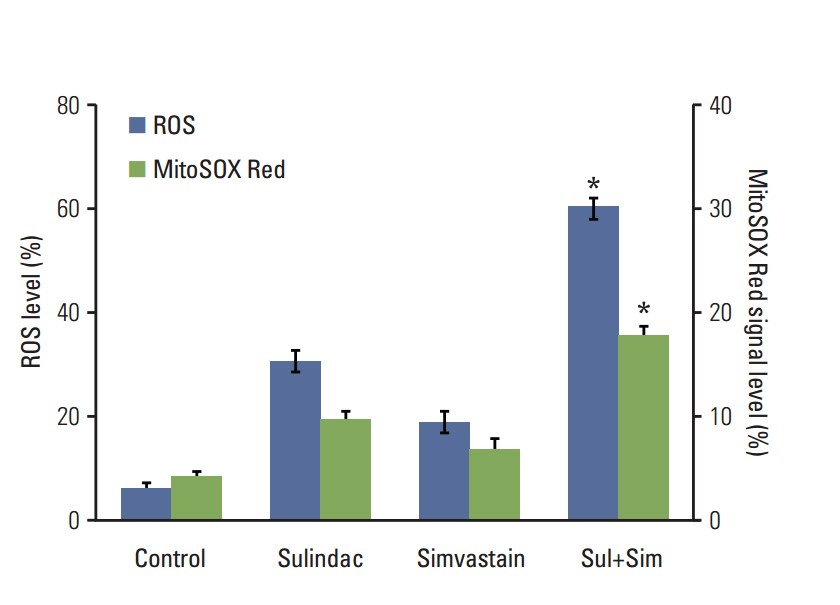
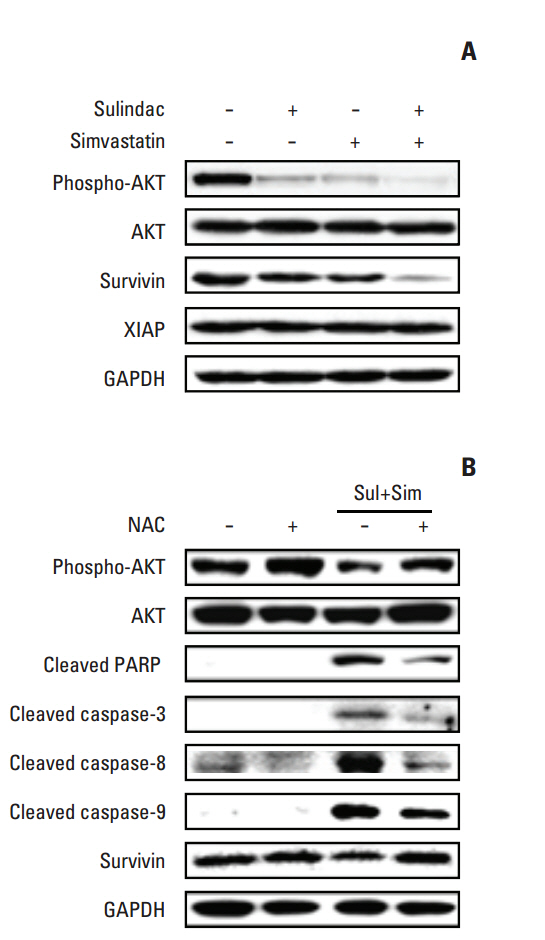
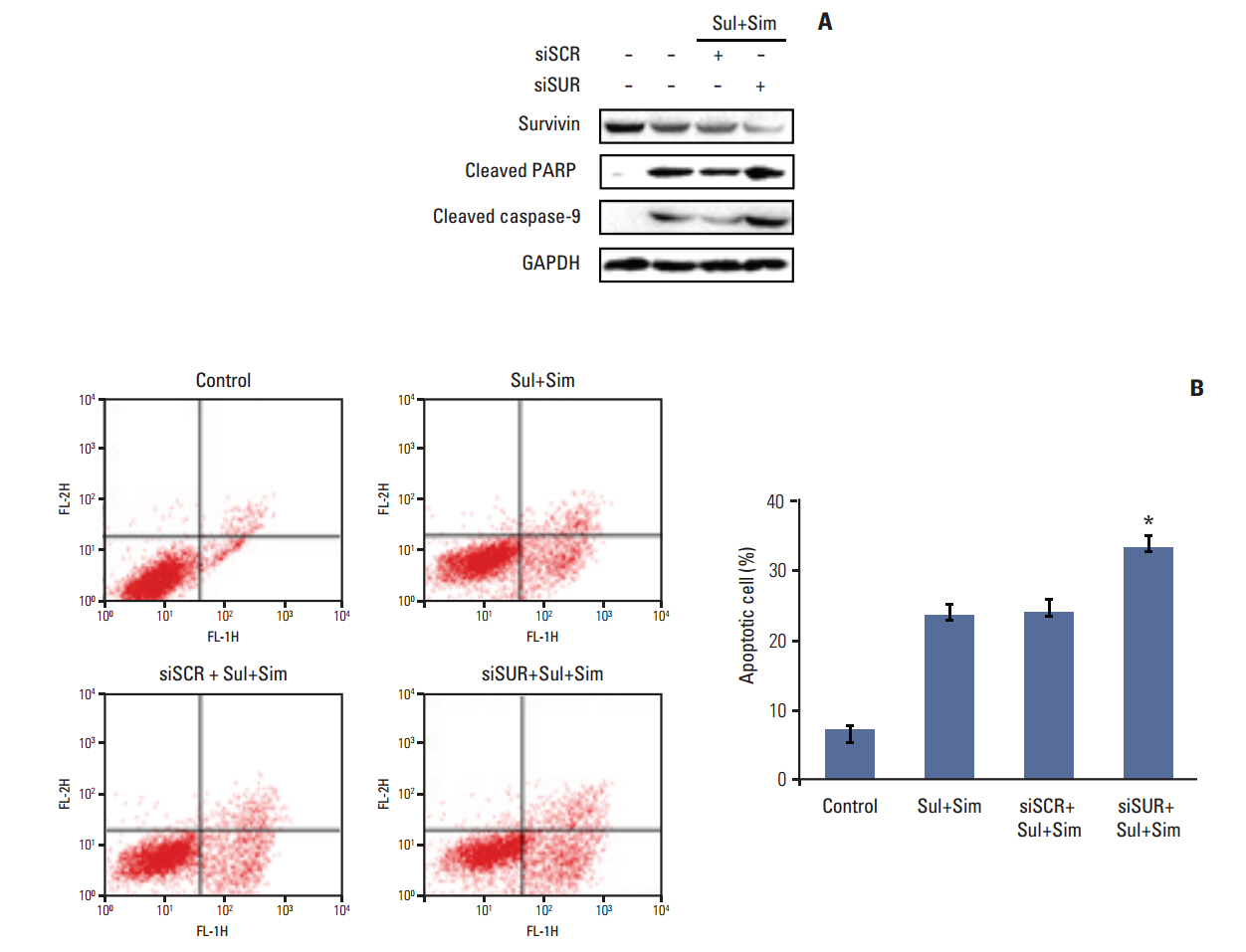
Cell viability was measured by an MTT assay, while the expression of apoptotic markers, AKT, and survivin in response to sulindac and simvastatin was examined by Western blotting. DNA fragmentation by apoptosis was analyzed by flow cytometry in A549 cells. Reactive oxygen species (ROS) generation was measured by flow cytometry using H2DCFDA and MitoSOX Red, and the effects of pretreatment with N-acetylcysteine were tested. The effects of AKT on survivin expression in sulindac- and simvastatin-treated cells were assessed. Survivin was knocked down or overexpressed to determine its role in apoptosis induced by sulindac and simvastatin.
RESULTS
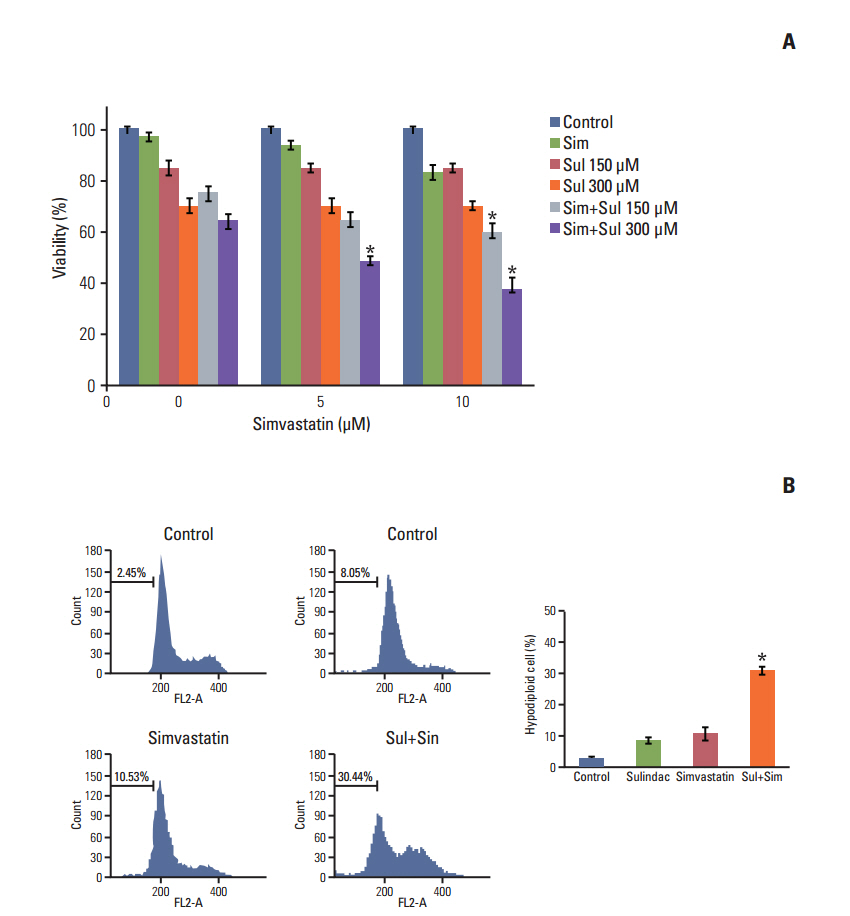
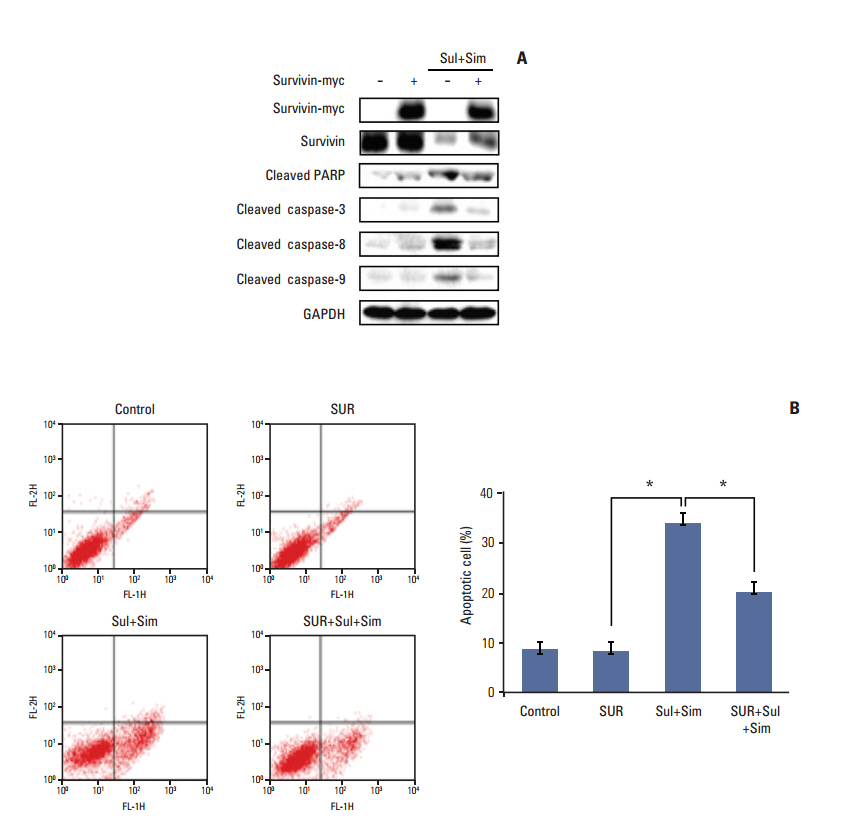
Sulindac and simvastatin synergistically augmented apoptotic activity and intracellular ROS production in A549 cells. Inhibition of AKT by siRNA or LY294002 inhibited survivin, while AKT overexpression markedly increased survivin expression, even in the presence of sulindac and simvastatin. Moreover, survivin siRNA enhanced sulindac- and simvastatininduced apoptosis. In contrast, survivin upregulation protected against sulindac- and simvastatin-induced apoptosis.
CONCLUSION
Combined treatment with sulindac and simvastatin augmented their apoptotic potential in lung cancer cells through AKT signaling-dependent downregulation of survivin. These results indicate that sulindac and simvastatin may be clinically promising therapies for the prevention of lung cancer.
Keyword
MeSH Terms
-
Acetylcysteine
Anti-Inflammatory Agents, Non-Steroidal
Apoptosis*
Blotting, Western
Carcinoma, Non-Small-Cell Lung
Cell Survival
DNA Fragmentation
Down-Regulation*
Flow Cytometry
Humans
Hydroxymethylglutaryl-CoA Reductase Inhibitors
Lung Neoplasms*
Oncogene Protein v-akt
Reactive Oxygen Species
RNA, Small Interfering
Simvastatin*
Sulindac*
Up-Regulation
Acetylcysteine
Anti-Inflammatory Agents, Non-Steroidal
Oncogene Protein v-akt
RNA, Small Interfering
Reactive Oxygen Species
Simvastatin
Sulindac
Figure
Reference
-
References
1. Omenn GS. Chemoprevention of lung cancer is proving difficult and frustrating, requiring new approaches. J Natl Cancer Inst. 2000; 92:959–60.
Article2. Narayanan BA, Narayanan NK, Pittman B, Reddy BS. Regression of mouse prostatic intraepithelial neoplasia by nonsteroidal anti-inflammatory drugs in the transgenic adenocarcinoma mouse prostate model. Clin Cancer Res. 2004; 10:7727–37.
Article3. Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer. 2006; 6:130–40.
Article4. Albayrak A, Polat B, Cadirci E, Hacimuftuoglu A, Halici Z, Gulapoglu M, et al. Gastric anti-ulcerative and anti-inflammatory activity of metyrosine in rats. Pharmacol Rep. 2010; 62:113–9.
Article5. Krysan K, Reckamp KL, Sharma S, Dubinett SM. The potential and rationale for COX-2 inhibitors in lung cancer. Anticancer Agents Med Chem. 2006; 6:209–20.
Article6. Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005; 352:1071–80.
Article7. Psaty BM, Potter JD. Risks and benefits of celecoxib to prevent recurrent adenomas. N Engl J Med. 2006; 355:950–2.
Article8. Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990; 343:425–30.
Article9. Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003; 9:10–9.10. Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005; 5:930–42.
Article11. Cafforio P, Dammacco F, Gernone A, Silvestris F. Statins activate the mitochondrial pathway of apoptosis in human lymphoblasts and myeloma cells. Carcinogenesis. 2005; 26:883–91.
Article12. Jalving M, Koornstra JJ, De Jong S, De Vries EG, Kleibeuker JH. Review article: the potential of combinational regimen with non-steroidal anti-inflammatory drugs in the chemoprevention of colorectal cancer. Aliment Pharmacol Ther. 2005; 21:321–39.
Article13. Hwang KE, Park C, Kwon SJ, Kim YS, Park DS, Lee MK, et al. Synergistic induction of apoptosis by sulindac and simvastatin in A549 human lung cancer cells via reactive oxygen speciesdependent mitochondrial dysfunction. Int J Oncol. 2013; 43:262–70.
Article14. Crissman HA, Steinkamp JA. Cell cycle-related changes in chromatin structure detected by flow cytometry using multiple DNA fluorochromes. Eur J Histochem. 1993; 37:129–38.15. Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001; 411:494–8.
Article16. Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998; 58:5315–20.17. Krajewska M, Krajewski S, Banares S, Huang X, Turner B, Bubendorf L, et al. Elevated expression of inhibitor of apoptosis proteins in prostate cancer. Clin Cancer Res. 2003; 9:4914–25.18. Minami T, Adachi M, Kawamura R, Zhang Y, Shinomura Y, Imai K. Sulindac enhances the proteasome inhibitor bortezomib-mediated oxidative stress and anticancer activity. Clin Cancer Res. 2005; 11:5248–56.
Article19. Mukhopadhyay P, Rajesh M, Yoshihiro K, Hasko G, Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun. 2007; 358:203–8.
Article20. Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, et al. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998; 396:580–4.
Article21. Kaneko R, Tsuji N, Asanuma K, Tanabe H, Kobayashi D, Watanabe N. Survivin down-regulation plays a crucial role in 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitorinduced apoptosis in cancer. J Biol Chem. 2007; 282:19273–81.
Article22. Ohashi H, Takagi H, Oh H, Suzuma K, Suzuma I, Miyamoto N, et al. Phosphatidylinositol 3-kinase/Akt regulates angiotensin II-induced inhibition of apoptosis in microvascular endothelial cells by governing survivin expression and suppression of caspase-3 activity. Circ Res. 2004; 94:785–93.
Article23. Fornaro M, Plescia J, Chheang S, Tallini G, Zhu YM, King M, et al. Fibronectin protects prostate cancer cells from tumor necrosis factor-alpha-induced apoptosis via the AKT/survivin pathway. J Biol Chem. 2003; 278:50402–11.24. Vaira V, Lee CW, Goel HL, Bosari S, Languino LR, Altieri DC. Regulation of survivin expression by IGF-1/mTOR signaling. Oncogene. 2007; 26:2678–84.
Article25. You L, He B, Xu Z, Uematsu K, Mazieres J, Mikami I, et al. Inhibition of Wnt-2-mediated signaling induces programmed cell death in non-small-cell lung cancer cells. Oncogene. 2004; 23:6170–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Growth Inhibition and Apoptosis Induction of Sulindac on Human Lung Cancer Cells
- Effect of Lactacystin on the Sulindac-Induced Apoptosis Mechanisms in HT-29 Cells
- Synergistic Cytotoxicity of Arsenic Trioxide and Sulindac Against Neuroblastoma
- Synergistic effect of ERK inhibition on tetrandrine-induced apoptosis in A549 human lung carcinoma cells
- Vanillin oxime inhibits lung cancer cell proliferation and activates apoptosis through JNK/ERK-CHOP pathway