Cancer Res Treat.
2023 Oct;55(4):1077-1086. 10.4143/crt.2022.1630.
Establishment of Patient-Derived Organoids Using Ascitic or Pleural Fluid from Cancer Patients
- Affiliations
-
- 1Center for Clinical Trials, National Cancer Center, Goyang, Korea
- 2Division of Cancer Biology, Cancer Molecular Biology Branch, Research Institute of National Cancer Center, Goyang, Korea
- 3Department of Cancer Biomedical Science, National Cancer Center Graduate School of Cancer Science and Policy, Goyang, Korea
- 4Division of Technology Convergence, Cancer Molecular Imaging Branch, Research Institute of National Cancer Center, Goyang, Korea
- 5Division of Rare and Refractory Cancer, Immuno-oncology Branch, Research Institute of National Cancer Center, Goyang, Korea
- 6Center for Liver and Pancreatobiliary Cancer, Hospital, National Cancer Center, Goyang, Korea
- 7Division of Rare and Refractory Cancer, Targeted Therapy Branch of Research Institute, National Cancer Center, Goyang, Korea
- 8Division of Clinical Research, Cancer Outcome & Quality Improvement Branch, Research Institute of National Cancer Center, Goyang, Korea
- 9Division of Clinical Research, Interventional Medicine Branch, Research Institute of National Cancer Center, Goyang, Korea
- 10Center for Breast Cancer, National Cancer Center, Goyang, Korea
- 11Division of Rare and Refractory Cancer, Anticancer Resistance Branch, Research Institute of National Cancer Center, Goyang, Korea
- 12Center for Cancer Data Center, National Cancer Control Institute of National Cancer Center, Goyang, Korea
- 13Department of Laboratory Medicine, National Cancer Center, Goyang, Korea
- KMID: 2547782
- DOI: http://doi.org/10.4143/crt.2022.1630
Abstract
- Purpose
Patient-derived tumor cells can be a powerful resource for studying pathophysiological mechanisms and developing robust strategies for precision medicine. However, establishing organoids from patient-derived cells is challenging because of limited access to tissue specimens. Therefore, we aimed to establish organoids from malignant ascites and pleural effusions.
Materials and Methods
Ascitic or pleural fluid from pancreatic, gastric, and breast cancer patients was collected and concentrated to culture tumor cells ex vivo. Organoids were considered to be successfully cultured when maintained for five or more passages. Immunohistochemical staining was performed to compare the molecular features, and drug sensitivity was assayed to analyze the clinical responses of original patients.
Results
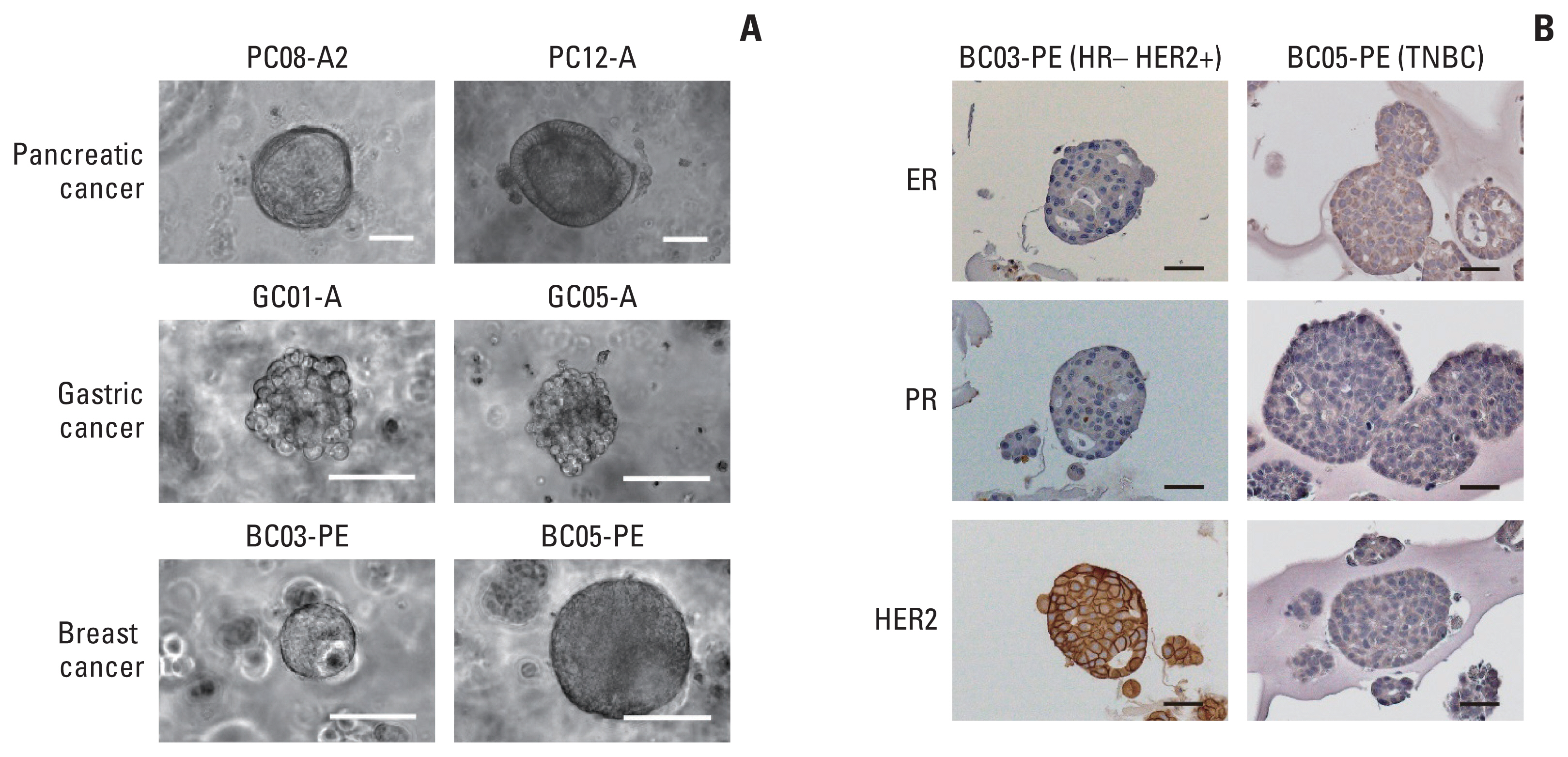
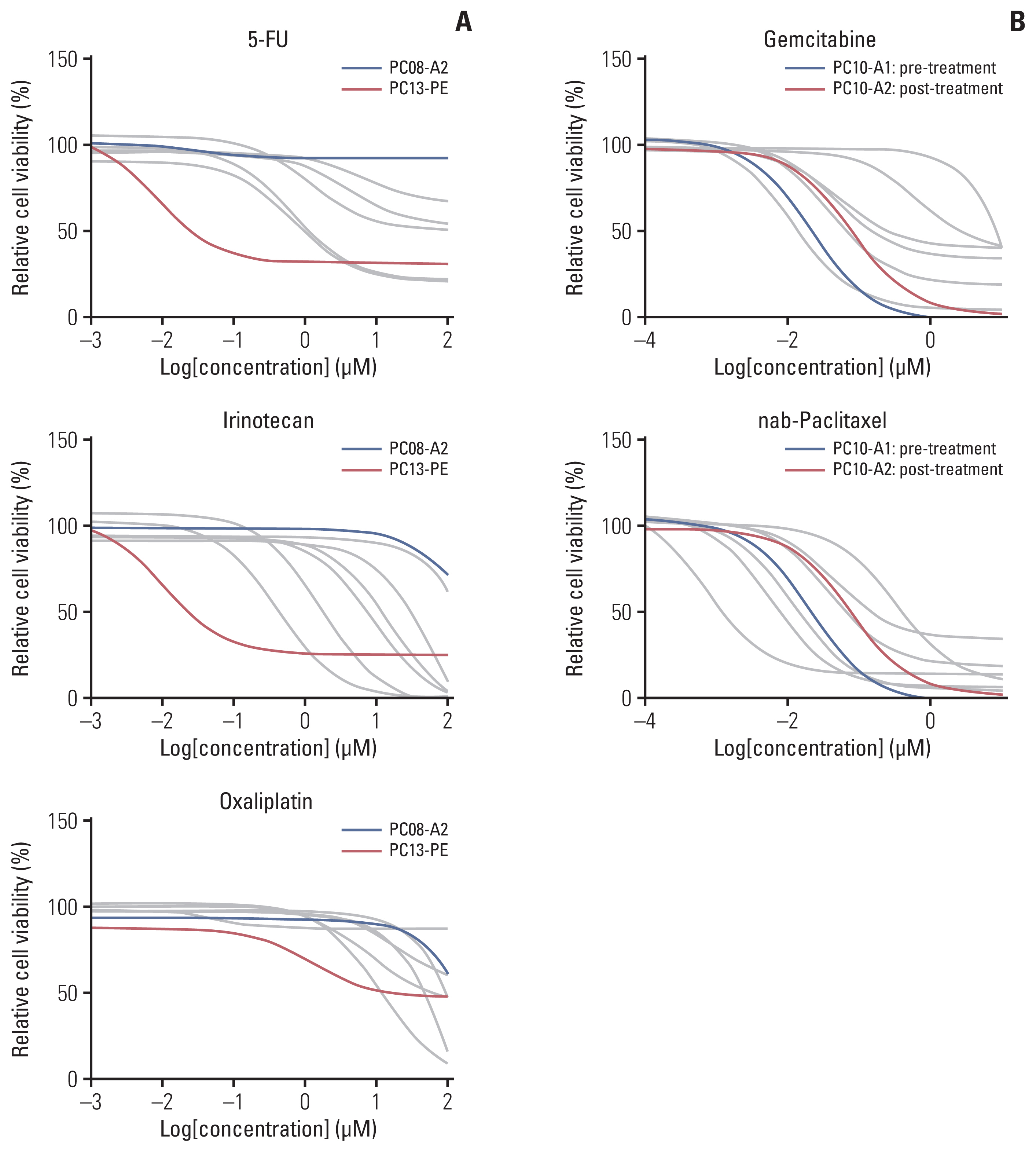
We collected 70 fluid samples from 58 patients (pancreatic cancer, n=39; gastric cancer, n=21; and breast cancer, n=10). The overall success rate was 40%; however, it differed with types of malignancy, with pancreatic, gastric, and breast cancers showing 48.7%, 33.3%, and 20%, respectively. Cytopathological results significantly differed between successful and failed cases (p=0.014). Immunohistochemical staining of breast cancer organoids showed molecular features identical to those of tumor tissues. In drug sensitivity assays, pancreatic cancer organoids recapitulated the clinical responses of the original patients.
Conclusion
Tumor organoids established from malignant ascites or pleural effusion of pancreatic, gastric, and breast cancers reflect the molecular characteristics and drug sensitivity profiles. Our organoid platform could be used as a testbed for patients with pleural and peritoneal metastases to guide precision oncology and drug discovery.
Keyword
Figure
Reference
-
References
1. Rijken A, Lurvink RJ, Luyer MD, Nieuwenhuijzen GA, van Erning FN, van Sandick JW, et al. The burden of peritoneal metastases from gastric cancer: a systematic review on the incidence, risk factors and survival. J Clin Med. 2021; 10:4882.2. Rijken A, Bakkers C, van Erning FN, van der Geest LG, de Vos-Geelen J, Besselink MG, et al. Incidence, treatment, and survival of synchronous peritoneal metastases in pancreatic cancer: update of a nationwide cohort. Pancreas. 2021; 50:827–33.3. Gayen S. Malignant pleural effusion: presentation, diagnosis, and management. Am J Med. 2022; 135:1188–92.4. Sun F, Feng M, Guan W. Mechanisms of peritoneal dissemination in gastric cancer. Oncol Lett. 2017; 14:6991–8.5. Avula LR, Hagerty B, Alewine C. Molecular mediators of peritoneal metastasis in pancreatic cancer. Cancer Metastasis Rev. 2020; 39:1223–43.6. Yarema R, Ohorchak M, Hyrya P, Kovalchuk Y, Safiyan V, Karelin I, et al. Gastric cancer with peritoneal metastases: efficiency of standard treatment methods. World J Gastrointest Oncol. 2020; 12:569–81.7. Thomassen I, Lemmens VE, Nienhuijs SW, Luyer MD, Klaver YL, de Hingh IH. Incidence, prognosis, and possible treatment strategies of peritoneal carcinomatosis of pancreatic origin: a population-based study. Pancreas. 2013; 42:72–5.8. Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. 2020; 21:571–84.9. Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F, et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018; 172:373–86.10. Yan HH, Siu HC, Law S, Ho SL, Yue SS, Tsui WY, et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell. 2018; 23:882–97.11. Driehuis E, van Hoeck A, Moore K, Kolders S, Francies HE, Gulersonmez MC, et al. Pancreatic cancer organoids recapitulate disease and allow personalized drug screening. Proc Natl Acad Sci U S A. 2019; 116:26580–90.12. Choi SI, Jeon AR, Kim MK, Lee YS, Im JE, Koh JW, et al. Development of patient-derived preclinical platform for metastatic pancreatic cancer: PDOX and a subsequent organoid model system using percutaneous biopsy samples. Front Oncol. 2019; 9:875.13. Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernandez-Mateos J, Khan K, et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science. 2018; 359:920–6.14. Ooft SN, Weeber F, Dijkstra KK, McLean CM, Kaing S, van Werkhoven E, et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci Transl Med. 2019; 11:eaay2574.15. Chen P, Zhang X, Ding R, Yang L, Lyu X, Zeng J, et al. Patient-derived organoids can guide personalized-therapies for patients with advanced breast cancer. Adv Sci (Weinh). 2021; 8:e2101176.16. Zheng LN, Wen F, Xu P, Zhang S. Prognostic significance of malignant ascites in gastric cancer patients with peritoneal metastasis: a systemic review and meta-analysis. World J Clin Cases. 2019; 7:3247–58.17. Baretti M, Pulluri B, Tsai HL, Blackford AL, Wolfgang CL, Laheru D, et al. The significance of ascites in patients with pancreatic ductal adenocarcinoma: a case-control study. Pancreas. 2019; 48:585–9.18. Tsai S, McOlash L, Palen K, Johnson B, Duris C, Yang Q, et al. Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models. BMC Cancer. 2018; 18:335.19. Li J, Xu H, Zhang L, Song L, Feng D, Peng X, et al. Malignant ascites-derived organoid (MADO) cultures for gastric cancer in vitro modelling and drug screening. J Cancer Res Clin Oncol. 2019; 145:2637–47.20. Pan B, Zhao D, Liu Y, Li N, Song C, Li N, et al. Breast cancer organoids from malignant pleural effusion-derived tumor cells as an individualized medicine platform. In Vitro Cell Dev Biol Anim. 2021; 57:510–8.21. Ponten F, Jirstrom K, Uhlen M. The Human Protein Atlas: a tool for pathology. J Pathol. 2008; 216:387–93.22. Ruschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, et al. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012; 25:637–50.23. Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009; 101:736–50.24. Cancer Genome Atlas Research Network. Integrated genomic characterization of pancreatic ductal adenocarcinoma. Cancer Cell. 2017; 32:185–203.25. Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011; 364:1817–25.26. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013; 369:1691–703.27. Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007; 25:1960–6.28. Mo S, Tang P, Luo W, Zhang L, Li Y, Hu X, et al. Patient-derived organoids from colorectal cancer with paired liver metastasis reveal tumor heterogeneity and predict response to chemotherapy. Adv Sci (Weinh). 2022; 9:e2204097.29. Yao Y, Xu X, Yang L, Zhu J, Wan J, Shen L, et al. Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell. 2020; 26:17–26.30. DeWitt J, Yu M, Al-Haddad MA, Sherman S, McHenry L, Leblanc JK. Survival in patients with pancreatic cancer after the diagnosis of malignant ascites or liver metastases by EUS-FNA. Gastrointest Endosc. 2010; 71:260–5.31. Acheampong T, Kehm RD, Terry MB, Argov EL, Tehranifar P. Incidence trends of breast cancer molecular subtypes by age and race/ethnicity in the US From 2010 to 2016. JAMA Netw Open. 2020; 3:e2013226.32. Feng F, Liu J, Wang F, Zheng G, Wang Q, Liu S, et al. Prognostic value of differentiation status in gastric cancer. BMC Cancer. 2018; 18:865.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cytologic Analysis of Metastatic Malignant Tumor in Pleural and Ascitic Fluid
- The Progress and Clinical Application of Breast Cancer Organoids
- Two Cases of Extragenital Infection by Mycoplasma hominis
- Preclinical investigation of patient-derived cervical cancer organoids for precision medicine
- Postmenopausal Meigs' Syndrome in Elevated CA-125: A Case Report