J Korean Med Sci.
2023 Oct;38(40):e332. 10.3346/jkms.2023.38.e332.
Long-Term Outcome of Unilateral Acoustic Neuromas With or Without Hearing Loss: Over 10 Years and Beyond After Gamma Knife Radiosurgery
- Affiliations
-
- 1Department of Neurosurgery, Soonchunhyang University Seoul Hospital, Seoul, Korea
- 2Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea
- 3Department of Neurosurgery, Seoul National University Bundang Hospital, Seongnam, Korea
- 4Department of Neurosurgery, Chungnam National University Hospital, Daejeon, Korea
- 5Department of Neurosurgery, Gachon University Gil Medical Center, Incheon, Korea
- 6Department of Internal Medicine, School of Medicine, Chung-Ang University, Seoul, Korea
- 7Department of Biostatistics, Soonchunhyang University Seoul Hospital, Seoul, Korea
- 8Clinical Research Institute, Seoul National University Hospital, Seoul, Korea
- 9Hypoxia/Ischemia Disease Institute, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 10Advanced Institutes of Convergence Technology, Suwon, Korea
- KMID: 2546937
- DOI: http://doi.org/10.3346/jkms.2023.38.e332
Abstract
- Background
Since the long-term outcomes of 162 patients who underwent gamma knife radiosurgery (GKS) as an initial or adjuvant treatment for acoustic neuromas (ANs) with unilateral hearing loss were first reported in 1998, there has been no report of a comprehensive analysis of what has changed in GKS practice.
Methods
We performed a retrospective study of the long-term outcomes of 106 patients with unilateral sporadic ANs who underwent GKS as an initial treatment. The mean patient age was 50 years, and the mean initial tumor volume was 3.68 cm 3 (range, 0.10–23.30 cm 3 ). The median marginal tumor dose was 12.5 Gy (range, 8.0–15.0 Gy) and the median follow-up duration was 153 months (range, 120–216 months).
Results
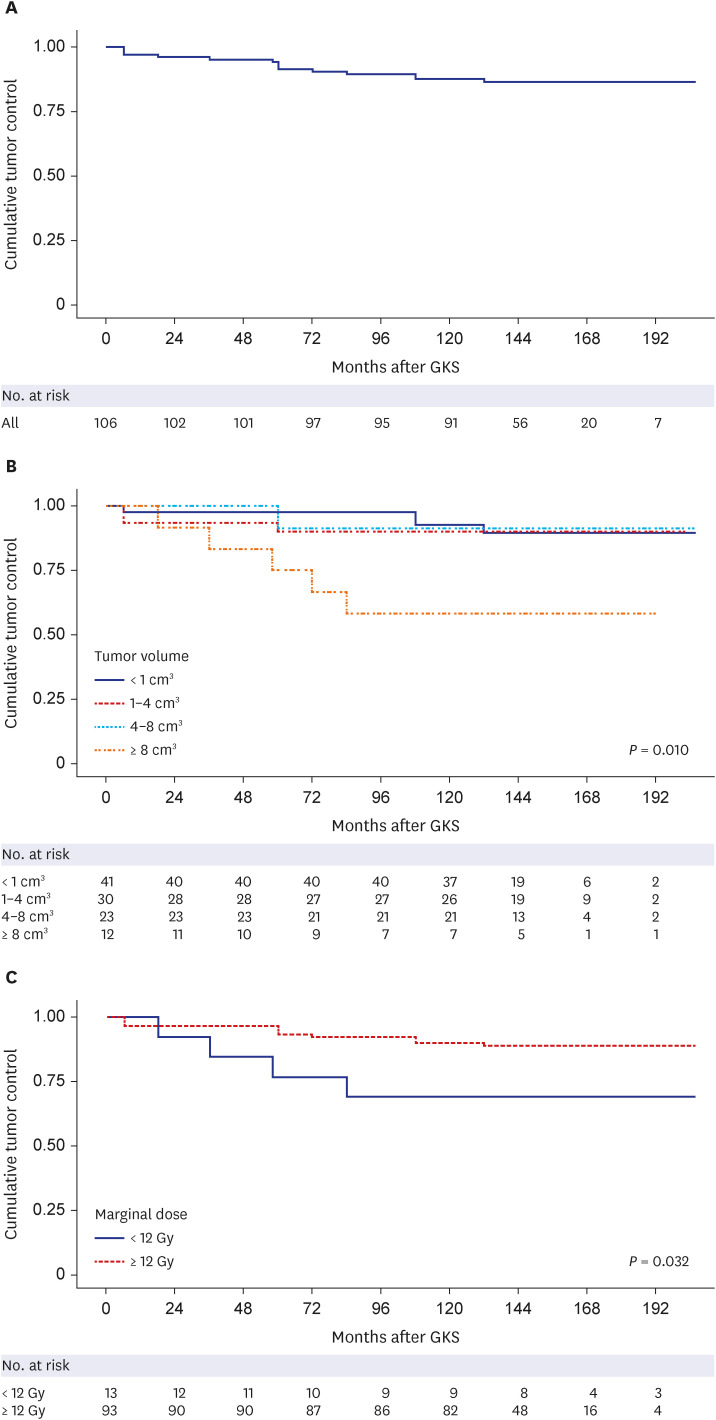
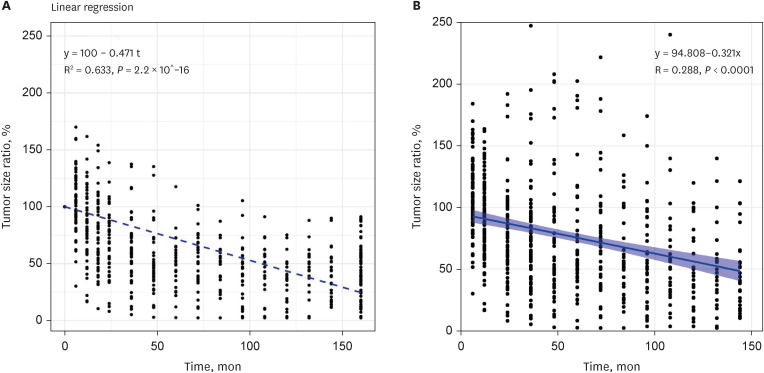
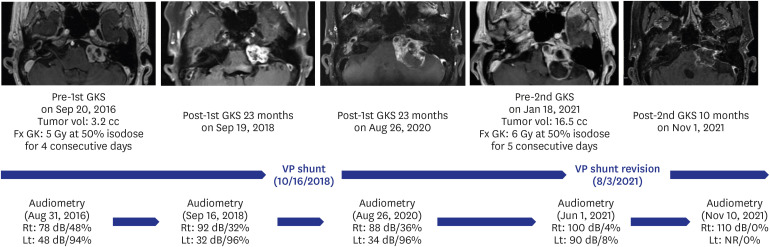
The tumor volume increased in 11 patients (10.4%), remained stationary in 27 (25.5%), and decreased in 68 patients (64.2%). The actuarial 3, 5, 10, and 15-year tumor control rates were 95.3 ± 2.1%, 94.3 ± 2.2%, 87.7 ± 3.2%, and 86.6 ± 3.3%, respectively. The 10-year actuarial tumor control rate was significantly lower in the patients with tumor volumes of ≥ 8 cm 3 (P = 0.010). The rate of maintaining the same Gardner-Robertson scale grade was 28.6%, and that of serviceable hearing was 46.4%. The rates of newly developed facial and trigeminal neuropathy were 2.8% and 4.7%, respectively. The patients who received marginal doses of less than 12 Gy revealed higher tumor control failure rates (P = 0.129) and newly occurred facial or trigeminal neuropathy rates (P = 0.040 and 0.313, respectively).
Conclusion
GKS as an initial treatment for ANs could be helpful in terms of tumor control, the preservation of serviceable hearing, and the prevention of cranial neuropathy. It is recommended to perform GKS as soon as possible not only for tumor control in unilateral ANs with hearing loss but also for hearing preservation in those without hearing loss.
Keyword
Figure
Reference
-
1. Oyler RF, Oyler AL, Matkin ND. Unilateral hearing loss: demographics and educational impact. Lang Speech Hear Serv Sch. 1988; 19(2):201–210.2. Charabi S, Tos M, Thomsen J, Charabi B, Mantoni M. Vestibular schwannoma growth--long-term results. Acta Otolaryngol Suppl. 2000; 543(543):7–10. PMID: 10908961.3. Deen HG, Ebersold MJ, Harner SG, Beatty CW, Marion MS, Wharen RE, et al. Conservative management of acoustic neuroma: an outcome study. Neurosurgery. 1996; 39(2):260–264. PMID: 8832662.4. Hoistad DL, Melnik G, Mamikoglu B, Battista R, O’Connor CA, Wiet RJ. Update on conservative management of acoustic neuroma. Otol Neurotol. 2001; 22(5):682–685. PMID: 11568679.5. Tschudi DC, Linder TE, Fisch U. Conservative management of unilateral acoustic neuromas. Am J Otol. 2000; 21(5):722–728. PMID: 10993466.6. Walsh RM, Bath AP, Bance ML, Keller A, Tator CH, Rutka JA. The natural history of untreated vestibular schwannomas. Is there a role for conservative management? Rev Laryngol Otol Rhinol (Bord). 2000; 121(1):21–26. PMID: 10865479.7. Son EI, Kim IM, Kim SP. Vestibular schwannoma with malignant transformation: a case report. J Korean Med Sci. 2001; 16(6):817–821. PMID: 11748371.8. Hirsch A, Norén G. Audiological findings after stereotactic radiosurgery in acoustic neurinomas. Acta Otolaryngol. 1988; 106(3-4):244–251. PMID: 3051887.9. Lee WJ, Lee JI, Choi JW, Kong DS, Nam DH, Cho YS, et al. Optimal volume of the residual tumor to predict long-term tumor control using stereotactic radiosurgery after facial nerve-preserving surgery for vestibular schwannomas. J Korean Med Sci. 2021; 36(16):e102. PMID: 33904259.10. Kim KM, Park CK, Chung HT, Paek SH, Jung HW, Kim DG. Long-term outcomes of gamma knife stereotactic radiosurgery of vestibular schwannomas. J Korean Neurosurg Soc. 2007; 42(4):286–292. PMID: 19096558.11. Kondziolka D, Lunsford LD, McLaughlin MR, Flickinger JC. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med. 1998; 339(20):1426–1433. PMID: 9811917.12. Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988; 97(1):55–66. PMID: 3277525.13. Norén G. Long-term complications following gamma knife radiosurgery of vestibular schwannomas. Stereotact Funct Neurosurg. 1998; 70(Suppl 1):65–73. PMID: 9782237.14. Yang I, Sughrue ME, Han SJ, Fang S, Aranda D, Cheung SW, et al. Facial nerve preservation after vestibular schwannoma Gamma Knife radiosurgery. J Neurooncol. 2009; 93(1):41–48. PMID: 19430881.15. Delsanti C, Tamura M, Galanaud D, Régis J. Changing radiological results, pitfalls and criteria of failure. Neurochirurgie. 2004; 50(2-3 Pt 2):312–319. PMID: 15179284.16. Pollock BE. Management of vestibular schwannomas that enlarge after stereotactic radiosurgery: treatment recommendations based on a 15 year experience. Neurosurgery. 2006; 58(2):241–248. PMID: 16462477.17. Kwon Y, Khang SK, Kim CJ, Lee DJ, Lee JK, Kwun BD. Radiologic and histopathologic changes after Gamma Knife radiosurgery for acoustic schwannoma. Stereotact Funct Neurosurg. 1999; 72(Suppl 1):2–10. PMID: 10681685.18. Nakamura H, Jokura H, Takahashi K, Boku N, Akabane A, Yoshimoto T. Serial follow-up MR imaging after gamma knife radiosurgery for vestibular schwannoma. AJNR Am J Neuroradiol. 2000; 21(8):1540–1546. PMID: 11003293.19. Norén G, Greitz D, Hirsch A, Lax I. Gamma knife surgery in acoustic tumours. Acta Neurochir Suppl (Wien). 1993; 58:104–107. PMID: 8109269.20. Yu CP, Cheung JY, Leung S, Ho R. Sequential volume mapping for confirmation of negative growth in vestibular schwannomas treated by gamma knife radiosurgery. J Neurosurg. 2000; 93(Suppl 3):82–89.21. Iwai Y, Yamanaka K, Shiotani M, Uyama T. Radiosurgery for acoustic neuromas: results of low-dose treatment. Neurosurgery. 2003; 53(2):282–287. PMID: 12925242.22. Flickinger JC, Kondziolka D, Niranjan A, Maitz A, Voynov G, Lunsford LD. Acoustic neuroma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2004; 60(1):225–230. PMID: 15337560.23. Sakamoto T, Fukuda S, Inuyama Y. Hearing loss and growth rate of acoustic neuromas in follow-up observation policy. Auris Nasus Larynx. 2001; 28(Suppl):S23–S27. PMID: 11683337.24. Zanoletti E, Mazzoni A, d’Avella D. Hearing preservation in small acoustic neuroma: observation or active therapy? Literature review and institutional experience. Acta Neurochir (Wien). 2019; 161(1):79–83. PMID: 30535851.25. Zanoletti E, Cazzador D, Faccioli C, Gallo S, Denaro L, D’Avella D, et al. Multi-option therapy vs observation for small acoustic neuroma: hearing-focused management. Acta Otorhinolaryngol Ital. 2018; 38(4):384–392. PMID: 30197430.26. Flickinger JC, Kondziolka D, Niranjan A, Lunsford LD. Results of acoustic neuroma radiosurgery: an analysis of 5 years’ experience using current methods. J Neurosurg. 2001; 94(1):1–6.27. Chopra R, Kondziolka D, Niranjan A, Lunsford LD, Flickinger JC. Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2007; 68(3):845–851. PMID: 17379451.28. Flickinger JC, Lunsford LD, Linskey ME, Duma CM, Kondziolka D. Gamma knife radiosurgery for acoustic tumors: multivariate analysis of four year results. Radiother Oncol. 1993; 27(2):91–98. PMID: 8356233.29. Flickinger JC, Kondziolka D, Niranjan A, Lunsford LD. Results of acoustic neuroma radiosurgery: an analysis of 5 years’ experience using current methods. J Neurosurg. 2001; 94(1):1–6.30. Flickinger JC, Kondziolka D, Niranjan A, Maitz A, Voynov G, Lunsford LD. Acoustic neuroma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2004; 60(1):225–230. PMID: 15337560.31. Lunsford LD, Niranjan A, Flickinger JC, Maitz A, Kondziolka D. Radiosurgery of vestibular schwannomas: summary of experience in 829 cases. J Neurosurg. 2005; 102(Suppl):195–199. PMID: 15662809.32. Chung WY, Liu KD, Shiau CY, Wu HM, Wang LW, Guo WY, et al. Gamma knife surgery for vestibular schwannoma: 10-year experience of 195 cases. J Neurosurg. 2005; 102(Suppl):87–96. PMID: 15662787.33. Myrseth E, Møller P, Pedersen PH, Vassbotn FS, Wentzel-Larsen T, Lund-Johansen M. Vestibular schwannomas: clinical results and quality of life after microsurgery or gamma knife radiosurgery. Neurosurgery. 2005; 56(5):927–935. PMID: 15854240.34. Chopra R, Kondziolka D, Niranjan A, Lunsford LD, Flickinger JC. Long-term follow-up of acoustic schwannoma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2007; 68(3):845–851. PMID: 17379451.35. Régis J, Tamura M, Delsanti C, Roche PH, Pellet W, Thomassin JM. Hearing preservation in patients with unilateral vestibular schwannoma after gamma knife surgery. Prog Neurol Surg. 2008; 21:142–151. PMID: 18810212.36. Yang I, Sughrue ME, Han SJ, Fang S, Aranda D, Cheung SW, et al. Facial nerve preservation after vestibular schwannoma Gamma Knife radiosurgery. J Neurooncol. 2009; 93(1):41–48. PMID: 19430881.37. Tamura M, Carron R, Yomo S, Arkha Y, Muraciolle X, Porcheron D, et al. Hearing preservation after gamma knife radiosurgery for vestibular schwannomas presenting with high-level hearing. Neurosurgery. 2009; 64(2):289–296. PMID: 19057423.38. Régis J, Carron R, Park MC, Soumare O, Delsanti C, Thomassin JM, et al. Wait-and-see strategy compared with proactive Gamma Knife surgery in patients with intracanalicular vestibular schwannomas. J Neurosurg. 2010; 113(Suppl):105–111. PMID: 21121792.39. Murphy ES, Barnett GH, Vogelbaum MA, Neyman G, Stevens GH, Cohen BH, et al. Long-term outcomes of Gamma Knife radiosurgery in patients with vestibular schwannomas. J Neurosurg. 2011; 114(2):432–440. PMID: 20095786.40. Breivik CN, Nilsen RM, Myrseth E, Pedersen PH, Varughese JK, Chaudhry AA, et al. Conservative management or gamma knife radiosurgery for vestibular schwannoma: tumor growth, symptoms, and quality of life. Neurosurgery. 2013; 73(1):48–56. PMID: 23615094.41. Kim YH, Kim DG, Han JH, Chung HT, Kim IK, Song SW, et al. Hearing outcomes after stereotactic radiosurgery for unilateral intracanalicular vestibular schwannomas: implication of transient volume expansion. Int J Radiat Oncol Biol Phys. 2013; 85(1):61–67. PMID: 22580122.42. Carlson ML, Jacob JT, Pollock BE, Neff BA, Tombers NM, Driscoll CL, et al. Long-term hearing outcomes following stereotactic radiosurgery for vestibular schwannoma: patterns of hearing loss and variables influencing audiometric decline. J Neurosurg. 2013; 118(3):579–587. PMID: 23101446.43. Hasegawa T, Kida Y, Kato T, Iizuka H, Kuramitsu S, Yamamoto T. Long-term safety and efficacy of stereotactic radiosurgery for vestibular schwannomas: evaluation of 440 patients more than 10 years after treatment with Gamma Knife surgery. J Neurosurg. 2013; 118(3):557–565. PMID: 23140152.44. Boari N, Bailo M, Gagliardi F, Franzin A, Gemma M, del Vecchio A, et al. Gamma Knife radiosurgery for vestibular schwannoma: clinical results at long-term follow-up in a series of 379 patients. J Neurosurg. 2014; 121(Suppl):123–142.45. Jacob JT, Carlson ML, Schiefer TK, Pollock BE, Driscoll CL, Link MJ. Significance of cochlear dose in the radiosurgical treatment of vestibular schwannoma: controversies and unanswered questions. Neurosurgery. 2014; 74(5):466–474. PMID: 24476904.46. Bir SC, Ambekar S, Bollam P, Nanda A. Long-term outcome of gamma knife radiosurgery for vestibular schwannoma. J Neurol Surg B Skull Base. 2014; 75(4):273–278. PMID: 25093151.47. Watanabe S, Yamamoto M, Kawabe T, Koiso T, Yamamoto T, Matsumura A, et al. Stereotactic radiosurgery for vestibular schwannomas: average 10-year follow-up results focusing on long-term hearing preservation. J Neurosurg. 2016; 125(Suppl 1):64–72. PMID: 27903183.48. Bowden G, Cavaleri J, Monaco E 3rd, Niranjan A, Flickinger J, Lunsford LD. Cystic vestibular schwannomas respond best to radiosurgery. Neurosurgery. 2017; 81(3):490–497. PMID: 28368501.49. Tuleasca C, Faouzi M, Maeder P, Maire R, Knisely J, Levivier M. Biologically effective dose correlates with linear tumor volume changes after upfront single-fraction stereotactic radiosurgery for vestibular schwannomas. Neurosurg Rev. 2021; 44(6):3527–3537. PMID: 33839944.50. Jones B, Hopewell JW, Paddick I. Biologically effective dose correlates with linear tumour volume changes after upfront single-fraction stereotactic radiosurgery for vestibular schwannomas. Neurosurg Rev. 2022; 45(3):2493–2495. PMID: 35290549.51. Savardekar AR, Terrell D, Lele SJ, Diaz R, Keesari PR, Trosclair K, et al. Primary treatment of small to medium (<3 cm) sporadic vestibular schwannomas: a systematic review and meta-analysis on hearing preservation and tumor control rates for microsurgery versus radiosurgery. World Neurosurg. 2022; 160:102–113.e12. PMID: 34838768.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Experience of Gamma Knife Rediosurgery for Acoustic Neurinomas
- A Case of Cochlear Implantation in Neurofibromatosis Type II
- Normal pressure hydrocephalus after gamma knife radiosurgery in a patient with vestibular schwannoma
- Gamma Knife Radiosurgery for Vestibular Schwannomas
- Hearing Preservation, Facial Nerve Dysfunction, and Tumor Control in Small Vestibular Schwannoma: A Systematic Review of Gamma Knife Radiosurgery Versus Microsurgery