Korean J Physiol Pharmacol.
2023 May;27(3):267-275. 10.4196/kjpp.2023.27.3.267.
New in vitro multiple cardiac ion channel screening system for preclinical Torsades de Pointes risk prediction under the Comprehensive in vitro Proarrhythmia Assay concepta
- Affiliations
-
- 1Pharmacological Research Division, Toxicological Evaluation and Research Department, National Institute of Food and Drug Safety Evaluation, Ministry of Food and Drug Safety, Cheongju 28159, Korea
- 2Department of Physiology, Kangwon National University School of Medicine, Chuncheon 24341, Korea
- KMID: 2541638
- DOI: http://doi.org/10.4196/kjpp.2023.27.3.267
Abstract
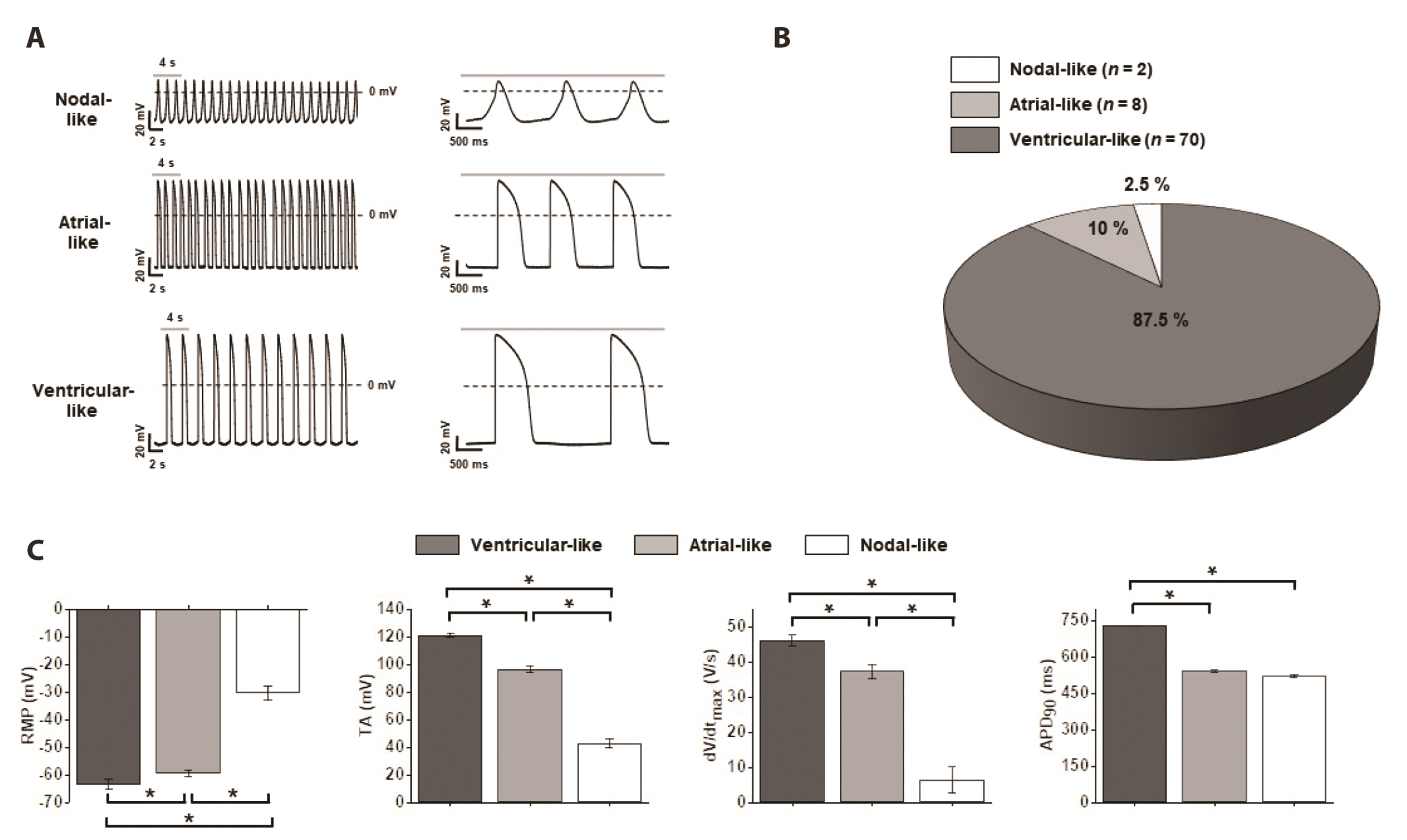
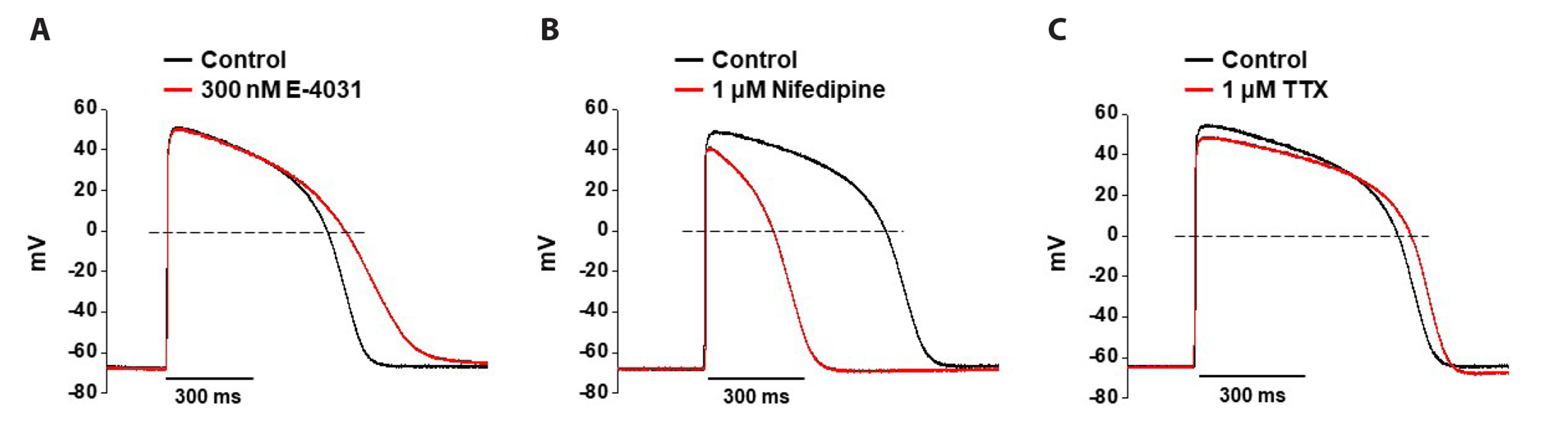
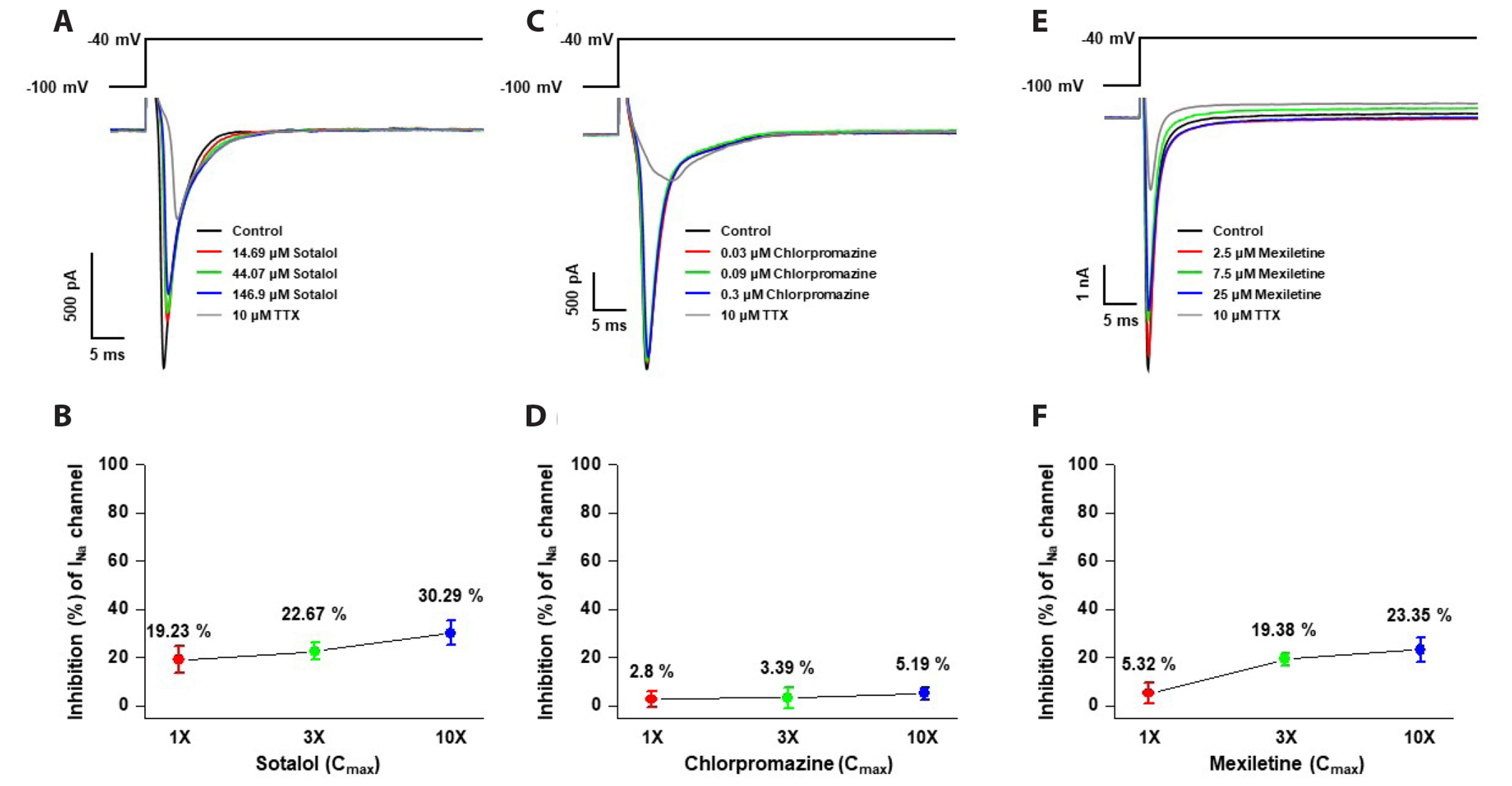
- Cardiotoxicity, particularly drug-induced Torsades de Pointes (TdP), is a concern in drug safety assessment. The recent establishment of human induced pluripotent stem cell-derived cardiomyocytes (human iPSC-CMs) has become an attractive human-based platform for predicting cardiotoxicity. Moreover, electrophysiological assessment of multiple cardiac ion channel blocks is emerging as an important parameter to recapitulate proarrhythmic cardiotoxicity. Therefore, we aimed to establish a novel in vitro multiple cardiac ion channel screening-based method using human iPSC-CMs to predict the drug-induced arrhythmogenic risk. To explain the cellular mechanisms underlying the cardiotoxicity of three representative TdP high- (sotalol), intermediate- (chlorpromazine), and low-risk (mexiletine) drugs, and their effects on the cardiac action potential (AP) waveform and voltage-gated ion channels were explored using human iPSC-CMs. In a proof-of-principle experiment, we investigated the effects of cardioactive channel inhibitors on the electrophysiological profile of human iPSC-CMs before evaluating the cardiotoxicity of these drugs. In human iPSC-CMs, sotalol prolonged the AP duration and reduced the total amplitude (TA) via selective inhibition of I Kr and I Na currents, which are associated with an increased risk of ventricular tachycardia TdP. In contrast, chlorpromazine did not affect the TA; however, it slightly increased AP duration via balanced inhibition of I Kr and I Ca currents. Moreover, mexiletine did not affect the TA, yet slightly reduced the AP duration via dominant inhibition of I Ca currents, which are associated with a decreased risk of ventricular tachycardia TdP. Based on these results, we suggest that human iPSC-CMs can be extended to other preclinical protocols and can supplement drug safety assessments.
Figure
Reference
-
1. Kannankeril PJ, Roden DM. 2007; Drug-induced long QT and torsade de pointes: recent advances. Curr Opin Cardiol. 22:39–43. DOI: 10.1097/HCO.0b013e32801129eb. PMID: 17143043.
Article2. Kola I, Landis J. 2004; Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 3:711–715. DOI: 10.1038/nrd1470. PMID: 15286737.
Article3. Food and Drug Administration, HHS. 2005; International Conference on Harmonisation; guidance on E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non-Antiarrhythmic Drugs; availability. Notice. Fed Regist. 70:61134–61135.4. Food and Drug Administration, HHS. 2005; International Conference on Harmonisation; guidance on S7B Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization (QT Interval Prolongation) by Human Pharmaceuticals; availability. Notice. Fed Regist. 70:61133–61134.5. Guo L, Coyle L, Abrams RM, Kemper R, Chiao ET, Kolaja KL. 2013; Refining the human iPSC-cardiomyocyte arrhythmic risk assessment model. Toxicol Sci. 136:581–594. DOI: 10.1093/toxsci/kft205. PMID: 24052561.
Article6. Cavero I, Holzgrefe H. 2014; Comprehensive in vitro Proarrhythmia Assay, a novel in vitro/in silico paradigm to detect ventricular proarrhythmic liability: a visionary 21st century initiative. Expert Opin Drug Saf. 13:745–758. DOI: 10.1517/14740338.2014.915311. PMID: 24845945.7. Sager PT, Gintant G, Turner JR, Pettit S, Stockbridge N. 2014; Rechanneling the cardiac proarrhythmia safety paradigm: a meeting report from the Cardiac Safety Research Consortium. Am Heart J. 167:292–300. DOI: 10.1016/j.ahj.2013.11.004. PMID: 24576511.
Article8. Magyar J, Iost N, Körtvély A, Bányász T, Virág L, Szigligeti P, Varró A, Opincariu M, Szécsi J, Papp JG, Nánási PP. 2000; Effects of endothelin-1 on calcium and potassium currents in undiseased human ventricular myocytes. Pflugers Arch. 441:144–149. DOI: 10.1007/s004240000400. PMID: 11205054.
Article9. Ando H, Yoshinaga T, Yamamoto W, Asakura K, Uda T, Taniguchi T, Ojima A, Shinkyo R, Kikuchi K, Osada T, Hayashi S, Kasai C, Miyamoto N, Tashibu H, Yamazaki D, Sugiyama A, Kanda Y, Sawada K, Sekino Y. 2017; A new paradigm for drug-induced torsadogenic risk assessment using human iPS cell-derived cardiomyocytes. J Pharmacol Toxicol Methods. 84:111–127. DOI: 10.1016/j.vascn.2016.12.003. PMID: 27956204.
Article10. Cavero I, Crumb W. 2005; ICH S7B draft guideline on the non-clinical strategy for testing delayed cardiac repolarisation risk of drugs: a critical analysis. Expert Opin Drug Saf. 4:509–530. DOI: 10.1517/14740338.4.3.509. PMID: 15934857.
Article11. Giorgi MA, Bolaños R, Gonzalez CD, Di Girolamo G. 2010; QT interval prolongation: preclinical and clinical testing arrhythmogenesis in drugs and regulatory implications. Curr Drug Saf. 5:54–57. DOI: 10.2174/157488610789869148. PMID: 20210719.
Article12. Lu HR, Mariën R, Saels A, De Clerck F. 2001; Species plays an important role in drug-induced prolongation of action potential duration and early afterdepolarizations in isolated Purkinje fibers. J Cardiovasc Electrophysiol. 12:93–102. DOI: 10.1046/j.1540-8167.2001.00093.x. PMID: 11204092.
Article13. Bussek A, Wettwer E, Christ T, Lohmann H, Camelliti P, Ravens U. 2009; Tissue slices from adult mammalian hearts as a model for pharmacological drug testing. Cell Physiol Biochem. 24:527–536. DOI: 10.1159/000257528. PMID: 19910693.
Article14. Thomas D, Wu K, Kathöfer S, Katus HA, Schoels W, Kiehn J, Karle CA. 2003; The antipsychotic drug chlorpromazine inhibits HERG potassium channels. Br J Pharmacol. 139:567–574. DOI: 10.1038/sj.bjp.0705283. PMID: 12788816. PMCID: PMC1573882.
Article15. Gualdani R, Tadini-Buoninsegni F, Roselli M, Defrenza I, Contino M, Colabufo NA, Lentini G. 2015; Inhibition of hERG potassium channel by the antiarrhythmic agent mexiletine and its metabolite m-hydroxymexiletine. Pharmacol Res Perspect. 3:e00160. DOI: 10.1002/prp2.160. PMID: 26516576. PMCID: PMC4618635.
Article16. Crumb WJ Jr, Vicente J, Johannesen L, Strauss DG. 2016; An evaluation of 30 clinical drugs against the comprehensive in vitro proarrhythmia assay (CiPA) proposed ion channel panel. J Pharmacol Toxicol Methods. 81:251–262. DOI: 10.1016/j.vascn.2016.03.009. PMID: 27060526.
Article17. Katchman AN, Koerner J, Tosaka T, Woosley RL, Ebert SN. 2006; Comparative evaluation of HERG currents and QT intervals following challenge with suspected torsadogenic and nontorsadogenic drugs. J Pharmacol Exp Ther. 316:1098–1106. DOI: 10.1124/jpet.105.093393. PMID: 16278312.
Article18. Gintant GA, Su Z, Martin RL, Cox BF. 2006; Utility of hERG assays as surrogate markers of delayed cardiac repolarization and QT safety. Toxicol Pathol. 34:81–90. DOI: 10.1080/01926230500431376. PMID: 16507548.
Article19. Belardinelli L, Liu G, Smith-Maxwell C, Wang WQ, El-Bizri N, Hirakawa R, Karpinski S, Li CH, Hu L, Li XJ, Crumb W, Wu L, Koltun D, Zablocki J, Yao L, Dhalla AK, Rajamani S, Shryock JC. 2013; A novel, potent, and selective inhibitor of cardiac late sodium current suppresses experimental arrhythmias. J Pharmacol Exp Ther. 344:23–32. DOI: 10.1124/jpet.112.198887. PMID: 23010360.
Article20. Johannesen L, Vicente J, Mason JW, Sanabria C, Waite-Labott K, Hong M, Guo P, Lin J, Sørensen JS, Galeotti L, Florian J, Ugander M, Stockbridge N, Strauss DG. 2014; Differentiating drug-induced multichannel block on the electrocardiogram: randomized study of dofetilide, quinidine, ranolazine, and verapamil. Clin Pharmacol Ther. 96:549–558. DOI: 10.1038/clpt.2014.155. PMID: 25054430.
Article21. Fermini B, Hancox JC, Abi-Gerges N, Bridgland-Taylor M, Chaudhary KW, Colatsky T, Correll K, Crumb W, Damiano B, Erdemli G, Gintant G, Imredy J, Koerner J, Kramer J, Levesque P, Li Z, Lindqvist A, Obejero-Paz CA, Rampe D, Sawada K, et al. 2016; A new perspective in the field of cardiac safety testing through the comprehensive in vitro Proarrhythmia Assay paradigm. J Biomol Screen. 21:1–11. DOI: 10.1177/1087057115594589. PMID: 26170255.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Torsades de Pointes Induced by Cisapride
- Introduction to in silico model for proarrhythmic risk assessment under the CiPA initiative
- Cardiac Arrest Related to Torsades de Pointes in a Patient Recovering from Diabetic Ketoacidosis
- A Case of Sudden Cardiac Death due to Pilsicainide-Induced Torsades de Pointes
- A Case of Hydroxyzine Induced Torsades de Pointes