J Stroke.
2023 Jan;25(1):160-168. 10.5853/jos.2022.02453.
The Rescue on Reperfusion Damage in Cerebral Infarction by Nelonemdaz (RODIN) Trial: Protocol for a Double-Blinded Clinical Trial of Nelonemdaz in Patients with Hyperacute Ischemic Stroke and Endovascular Thrombectomy
- Affiliations
-
- 1Department of Neurology, Ajou University School of Medicine, Ajou University Medical Center, Suwon, Korea
- 2Clinical Research Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3GNT Pharma Co., Ltd., Yongin, Korea
- 4Department of Neurology, Stony Brook University School of Medicine, New York, NY, USA
- 5Department of Neurology and Research Institute of Clinical Medicine of Jeonbuk National University, Jeonju, Korea
- 6Department of Neurology, Seoul Hospital, Ewha University College of Medicine, Seoul, Korea
- 7Department of Neurology, Chosun University College of Medicine, Gwangju, Korea
- 8Department of Neurology, Gyoungsang National University Hospital School of Medicine, Jinju, Korea
- 9partment of Neurology, Chungbuk National University Hospital, Chungbuk National University College of Medicine, Cheongju, Korea
- 10Department of Neurology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2539069
- DOI: http://doi.org/10.5853/jos.2022.02453
Abstract
- Background and Purpose
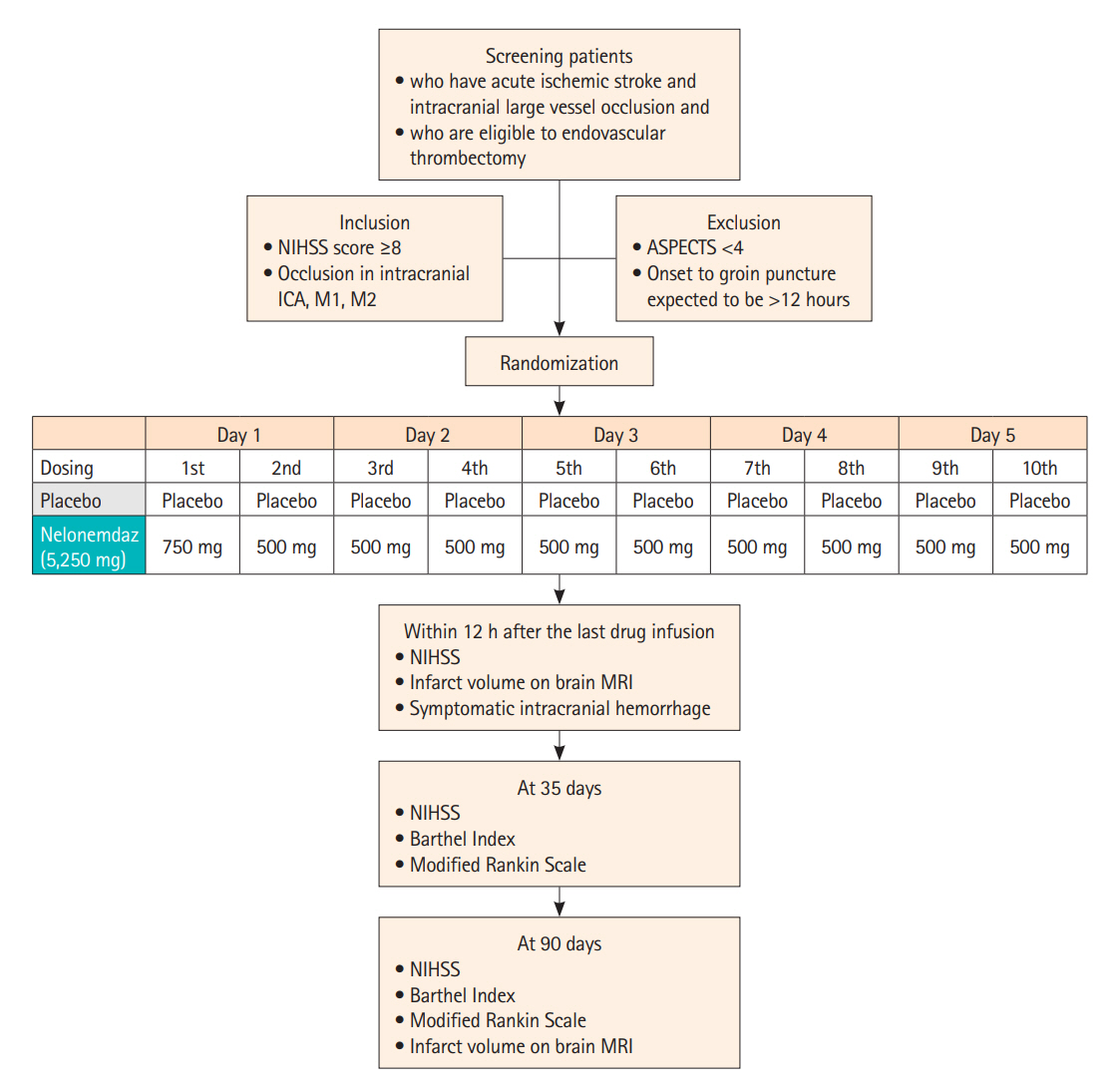
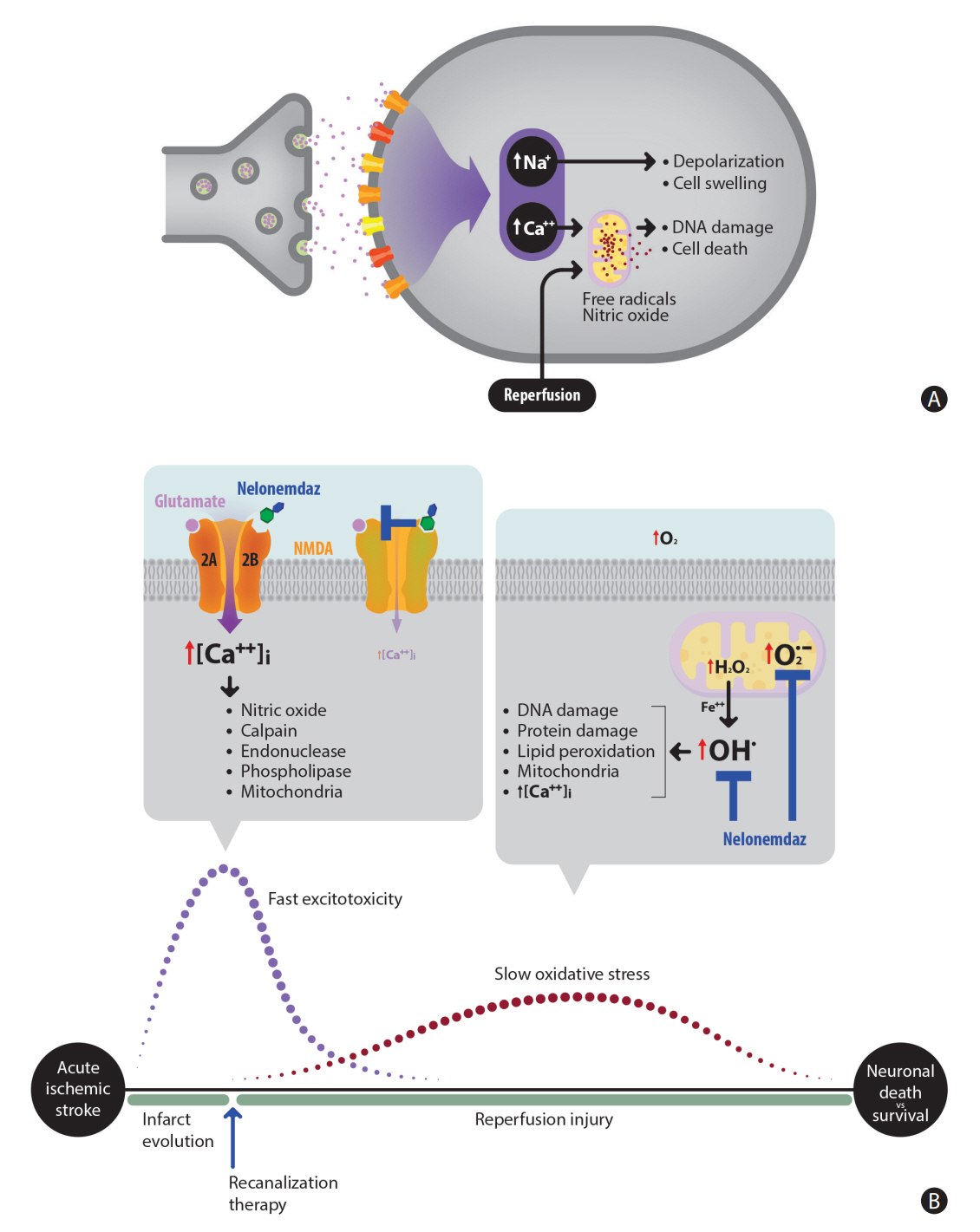
Nelonemdaz (Neu2000) has both selective antagonism against 2B subunit of N-methyl-D-aspartate receptor and antioxidant activity. This drug provides sufficient evidence of neuroprotection in acute cerebral ischemia/reperfusion models. This phase III trial aims to determine this effect in patients. Design The Rescue on Reperfusion Damage in Cerebral Infarction by Nelonemdaz is a multicenter, double-blinded clinical trial. A total of 496 patients will be randomly assigned into the nelonemdaz (a total of 5,250 mg divided by 10 times for 5 days) and placebo groups. Patients will be included if they have an acute ischemic stroke (National Institutes of Health Stroke Scale score ≥8) caused by intracranial large vessel occlusion in the anterior circulation (Alberta Stroke Program Early CT Score ≥4), and if they are expected to undergo endovascular thrombectomy within 12 hours after stroke onset. Endpoints The primary endpoint is a favorable shift in the modified Rankin Scale (mRS) score at 90 days after the first dose of drug. The data will be analyzed by the Cochran–Mantel–Haenszel shift test. The secondary endpoints include functional independence (mRS 0–2) at 35 and 90 days, the favorable shift of mRS at 35 days, the proportion of mRS 0 at 35 and 90 days, and the occurrence rates of symptomatic intracranial hemorrhage within 7 days.
Conclusion
This trial will clarify the efficacy and safety of nelonemdaz in patients with acute ischemic stroke and endovascular thrombectomy. This study has been registered at ClinicalTrials. gov (NCT05041010).
Keyword
Figure
Reference
-
References
1. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015; 372:11–20.2. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015; 372:1019–1030.3. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015; 372:2285–2295.4. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015; 372:2296–2306.5. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015; 372:1009–1018.6. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016; 387:1723–1731.7. Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999; 22:391–397.8. Wu QJ, Tymianski M. Targeting NMDA receptors in stroke: new hope in neuroprotection. Mol Brain. 2018; 11:15.9. Choi DW. Excitotoxicity: still hammering the ischemic brain in 2020. Front Neurosci. 2020; 14:579953.10. Gwag BJ, Lee YA, Ko SY, Lee MJ, Im DS, Yun BS, et al. Marked prevention of ischemic brain injury by Neu2000, an NMDA antagonist and antioxidant derived from aspirin and sulfasalazine. J Cereb Blood Flow Metab. 2007; 27:1142–1151.11. Noh J, Lee ES, Chung JM. The novel NMDA receptor antagonist, 2-hydroxy-5-(2,3,5,6-tetrafluoro-4-trifluoromethyl-benzylamino)-benzoic acid, is a gating modifier in cultured mouse cortical neurons. J Neurochem. 2009; 109:1261–1271.12. Cho SI, Park UJ, Chung JM, Gwag BJ. Neu2000, an NR2B-selective, moderate NMDA receptor antagonist and potent spin trapping molecule for stroke. Drug News Perspect. 2010; 23:549–556.13. Visavadiya NP, McEwen ML, Pandya JD, Sullivan PG, Gwag BJ, Springer JE. Antioxidant properties of Neu2000 on mitochondrial free radicals and oxidative damage. Toxicol In Vitro. 2013; 27:788–797.14. Im DS, Jeon JW, Lee JS, Won SJ, Cho SI, Lee YB, et al. Role of the NMDA receptor and iron on free radical production and brain damage following transient middle cerebral artery occlusion. Brain Res. 2012; 1455:114–123.15. Hong JM, Lee JS, Lee YB, Shin DH, Shin DI, Hwang YH, et al. Nelonemdaz for patients with acute ischemic stroke undergoing endovascular reperfusion therapy: a randomized phase II trial. Stroke. 2022; 53:3250–3259.16. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005; 3:692–694.17. Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007; 38:1091–1096.18. Quinn TJ, Langhorne P, Stott DJ. Barthel index for stroke trials: development, properties, and application. Stroke. 2011; 42:1146–1151.19. Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989; 20:864–870.20. Sutherland BA, Minnerup J, Balami JS, Arba F, Buchan AM, Kleinschnitz C. Neuroprotection for ischaemic stroke: translation from the bench to the bedside. Int J Stroke. 2012; 7:407–418.21. Lee JS, Lee SJ, Hong JM, Choi JW, Hong JH, Chang HW, et al. Temporal changes in care processes and outcomes for endovascular treatment of acute ischemic stroke: retrospective registry data from three Korean centers. Neurointervention. 2018; 13:2–12.22. Lee JS, Hwang YH, Sohn SI. Factors contributing to an efficacious endovascular treatment for acute ischemic stroke in Asian population. Neurointervention. 2021; 16:91–110.23. Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995; 52:998–1007.24. Gwag BJ, Lobner D, Koh JY, Wie MB, Choi DW. Blockade of glutamate receptors unmasks neuronal apoptosis after oxygen-glucose deprivation in vitro. Neuroscience. 1995; 68:615–619.25. Davis SM, Lees KR, Albers GW, Diener HC, Markabi S, Karlsson G, et al. Selfotel in acute ischemic stroke: possible neurotoxic effects of an NMDA antagonist. Stroke. 2000; 31:347–354.26. Albers GW, Goldstein LB, Hall D, Lesko LM; Aptiganel Acute Stroke Investigators. Aptiganel hydrochloride in acute ischemic stroke: a randomized controlled trial. JAMA. 2001; 286:2673–2682.27. Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002; 1:383–386.28. Hill MD, Goyal M, Menon BK, Nogueira RG, McTaggart RA, Demchuk AM, et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet. 2020; 395:878–887.29. Park UJ, Lee YA, Won SM, Lee JH, Kang SH, Springer JE, et al. Blood-derived iron mediates free radical production and neuronal death in the hippocampal CA1 area following transient forebrain ischemia in rat. Acta Neuropathol. 2011; 121:459–473.30. Hill MD. Efficacy and safety of nerinetide in participants with acute ischemic stroke undergoing endovascular thrombectomy excluding thrombolysis (ESCAPE-NEXT) [Internet]. Bethesda, MD: U.S. National Library of Medicine;2020. [cited 2022 Jul 14]. Available from: https://clinicaltrials.gov/ct2/show/NCT04462536.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Choosing a Hyperacute Stroke Imaging Protocol for Proper Patient Selection and Time Efficient Endovascular Treatment: Lessons from Recent Trials
- Guideline for intra-arterial thrombolysis of hyperacute ischemic stroke patients: Preliminary Report
- Surgical Management of Acute Stroke
- DIRECT-SAFE: A Randomized Controlled Trial of DIRECT Endovascular Clot Retrieval versus Standard Bridging Therapy
- Targeted temperature management for ischemic stroke