J Stroke.
2022 Jan;24(1):57-64. 10.5853/jos.2021.03475.
DIRECT-SAFE: A Randomized Controlled Trial of DIRECT Endovascular Clot Retrieval versus Standard Bridging Therapy
- Affiliations
-
- 1Department of Radiology, The Royal Melbourne Hospital, University of Melbourne, Parkville, Australia
- 2Department of Medicine and Neurology, Melbourne Brain Centre at The Royal Melbourne Hospital, University of Melbourne, Parkville, Australia
- 3Melbourne Medical School, University of Melbourne, Parkville, Australia
- 4Comprehensive Stroke Centre, Department of Neurology, The People’s Hospital 115, Pham Ngoc Thach University of Medicine, Ho Chi Minh City, Vietnam
- 5The Florey Institute of neuroscience and Mental Health, Parkville, Australia
- 6Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 7Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- KMID: 2525331
- DOI: http://doi.org/10.5853/jos.2021.03475
Abstract
- Background and Purpose
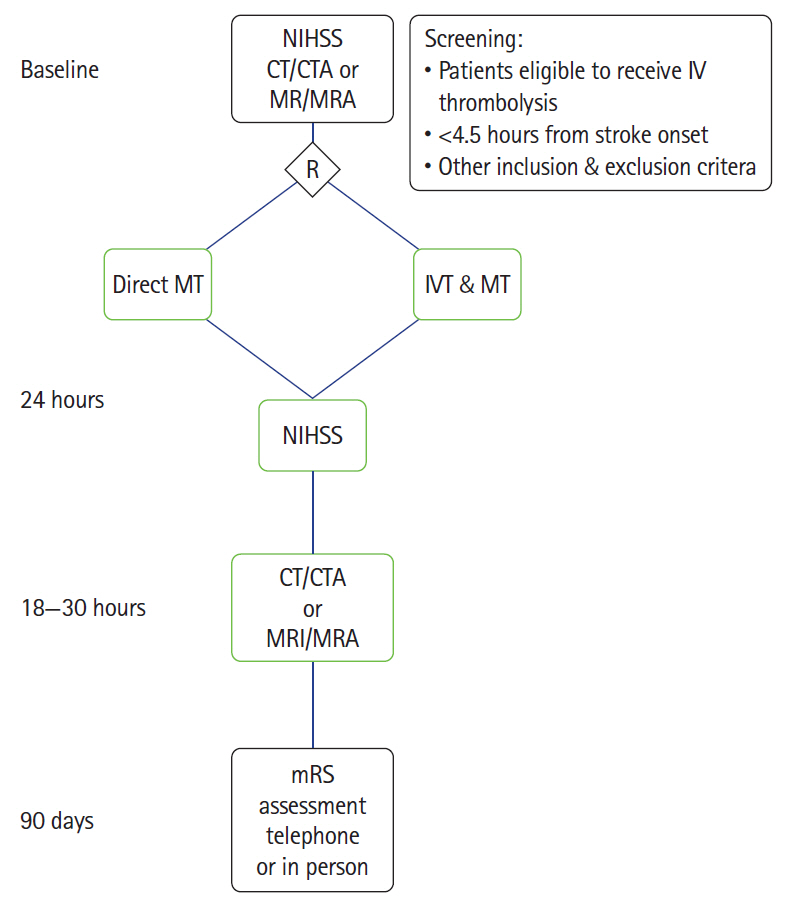
The benefit regarding co-treatment with intravenous (IV) thrombolysis before mechanical thrombectomy in acute ischemic stroke with large vessel occlusion remains unclear. To test the hypothesis that clinical outcome of ischemic stroke patients with intracranial internal carotid artery, middle cerebral artery or basilar artery occlusion treated with direct endovascular thrombectomy within 4.5 hours will be non-inferior compared with that of standard bridging IV thrombolysis followed by endovascular thrombectomy.
Methods
To randomize 780 patients 1:1 to direct thrombectomy or bridging IV thrombolysis with thrombectomy. An international-multicenter prospective randomized open label blinded endpoint trial (PROBE) (ClincalTrials.gov identifier: NCT03494920).
Results
Primary endpoint is functional independence defined as modified Rankin Scale (mRS) 0–2 or return to baseline at 90 days. Secondary end points include ordinal mRS analysis, good angiographic reperfusion (modified Thrombolysis in Cerebral Infarction score [mTICI] 2b–3), safety endpoints include symptomatic intracerebral hemorrhage and death.
Conclusions
DIRECT-SAFE will provide unique information regarding the impact of direct thrombectomy in patients with large vessel occlusion, including patients with basilar artery occlusion, with comparison across different ethnic groups.
Keyword
Figure
Reference
-
References
1. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016; 387:1723–1731.
Article2. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014; 384:1929–1935.
Article3. Ma H, Campbell B, Parsons MW, Churilov L, Levi CR, Hsu C, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019; 380:1795–1803.
Article4. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015; 372:11–20.5. Suzuki Y, Nagai N, Umemura K. A review of the mechanisms of blood-brain barrier permeability by tissue-type plasminogen activator treatment for cerebral ischemia. Front Cell Neurosci. 2016; 10:2.
Article6. Wang W, Li M, Chen Q, Wang J. Hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke: mechanisms, models, and biomarkers. Mol Neurobiol. 2015; 52:1572–1579.
Article7. Anderson CS, Robinson T, Lindley RI, Arima H, Lavados PM, Lee TH, et al. Low-dose versus standard-dose intravenous alteplase in acute ischemic stroke. N Engl J Med. 2016; 374:2313–2323.8. Gilgen MD, Klimek D, Liesirova KT, Meisterernst J, Klinger-Gratz PP, Schroth G, et al. Younger stroke patients with large pretreatment diffusion-weighted imaging lesions may benefit from endovascular treatment. Stroke. 2015; 46:2510–2516.
Article9. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015; 372:2285–2295.
Article10. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015; 372:1009–1018.
Article11. Coutinho JM, Liebeskind DS, Slater LA, Nogueira RG, Clark W, Dávalos A, et al. Combined intravenous thrombolysis and thrombectomy vs thrombectomy alone for acute ischemic stroke: a pooled analysis of the SWIFT and STAR studies. JAMA Neurol. 2017; 74:268–274.12. Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010; 41:2254–2258.13. McArthur KS, Johnson PC, Quinn TJ, Higgins P, Langhorne P, Walters MR, et al. Improving the efficiency of stroke trials: feasibility and efficacy of group adjudication of functional end points. Stroke. 2013; 44:3422–3428.14. Mehta CR, Pocock SJ. Adaptive increase in sample size when interim results are promising: a practical guide with examples. Stat Med. 2011; 30:3267–3284.
Article15. Churilov L, Arnup S, Johns H, Leung T, Roberts S, Campbell BC, et al. An improved method for simple, assumption-free ordinal analysis of the modified Rankin Scale using generalized odds ratios. Int J Stroke. 2014; 9:999–1005.
Article16. Howard G, Waller JL, Voeks JH, Howard VJ, Jauch EC, Lees KR, et al. A simple, assumption-free, and clinically interpretable approach for analysis of modified Rankin outcomes. Stroke. 2012; 43:664–669.
Article17. Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001; 134:663–694.
Article18. Yang P, Zhang Y, Zhang L, Zhang Y, Treurniet KM, Chen W, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020; 382:1981–1993.
Article19. Suzuki K, Matsumaru Y, Takeuchi M, Morimoto M, Kanazawa R, Takayama Y, et al. Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: the SKIP randomized clinical trial. JAMA. 2021; 325:244–253.20. Zi W, Qiu Z, Li F, Sang H, Wu D, Luo W, et al. Effect of endovascular treatment alone vs intravenous alteplase plus endovascular treatment on functional independence in patients with acute ischemic stroke: the DEVT randomized clinical trial. JAMA. 2021; 325:234–243.21. Roos Y. Direct endovascular treatment (dEVT) versus intravenous alteplase followed by endovascular treatment in patients with acute stroke due to a large vessel occlusion. In : Proceedings of the International Stroke Conference 2021; 2021 Mar 17-19; Online.
Article22. Fischer U, Gralla J. Solitaire™ With the Intention For Thrombectomy Plus Intravenous t-PA Versus DIRECT Solitaire™ Stent-retriever Thrombectomy in Acute Anterior Circulation Stroke (SWIFT DIRECT). In : Proceedings of the 7th European Stroke Conference; 2021 Sep 1-3; Online.
Article23. Lin CH, Saver JLZ, Ovbiagele B, Huang WY, Lee M. Endovascular thrombectomy without versus with intravenous thrombolysis in acute ischemic stroke: a non-inferiority meta-analysis of randomized clinical trials. J Neurointerv Surg. 2021; Jul. 15. [Epub]. https://doi.org/10.1136/neurintsurg-2021-017667.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evolution of Endovascular Therapy in Acute Stroke: Implications of Device Development
- Current Update on the Randomized Controlled Trials of Intracranial Aneurysms
- Endovascular Stroke Therapy Focused on Stent Retriever Thrombectomy and Direct Clot Aspiration: Historical Review and Modern Application
- Acute frame coil migration during filling coil retrieval in a cerebral aneurysm embolization case: A possible result of a venturi effect?
- A Less Invasive Approach for Ruptured Aneurysm with Intracranial Hematoma: Coil Embolization Followed by Clot Evacuation