J Korean Neurosurg Soc.
2022 Mar;65(2):204-214. 10.3340/jkns.2021.0098.
The Effect of Genetically Modified Lactobacillus plantarum Carrying Bone Morphogenetic Protein 2 Gene on an Ovariectomized Rat
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Kyung Hee University, Seoul, Korea
- 2Laboratory of Stem Cell Therapy, Asan Medical Center, College of Medicine, University of Ulsan, Seoul, Korea
- 3Department of Biotechnology, Korea National University of Transportation, Jeungpyeong, Korea
- 4Department of Neurological Surgery, Asan Medical Center, College of Medicine, University of Ulsan, Seoul, Korea
- 5Department of Rehabilitation Medicine, Asan Medical Center, College of Medicine, University of Ulsan, Seoul, Korea
- 6Department of Neurosurgery, Soonchunhyang University Bucheon Hospital, Bucheon, Korea
- KMID: 2527177
- DOI: http://doi.org/10.3340/jkns.2021.0098
Abstract
Objective
: Osteoporosis result from age-related decline in the number of osteoblast progenitors in the bone marrow. Probiotics have beneficial effects on the host, when administered in appropriate amounts. This study investigated the effects of probiotics expressing specific genes, especially the effects of genetically modified bone morphogenetic protein (BMP)-2-expressing Lactobacillus plantarum CJNU 3003 (LP) on ovariectomized rats.
Methods
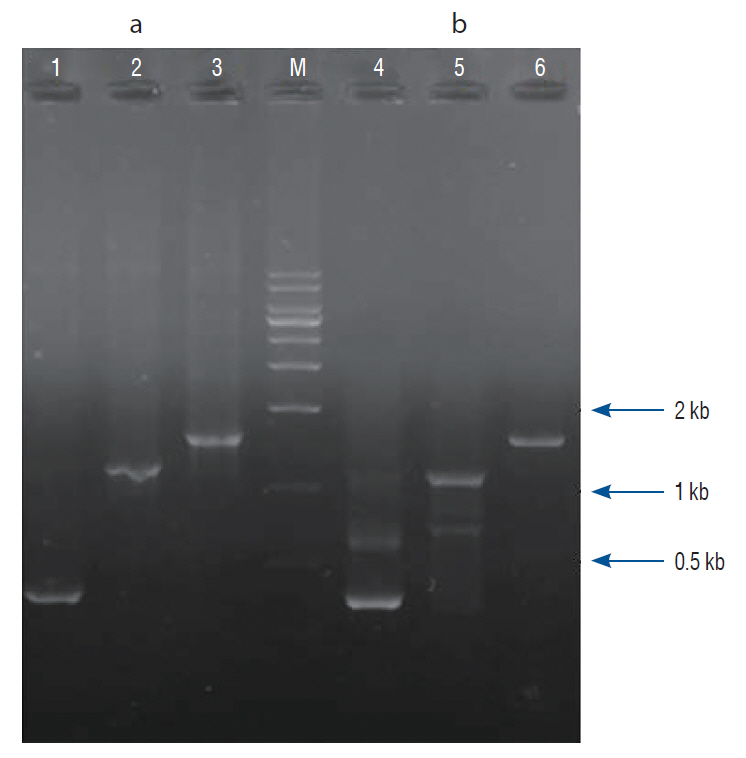
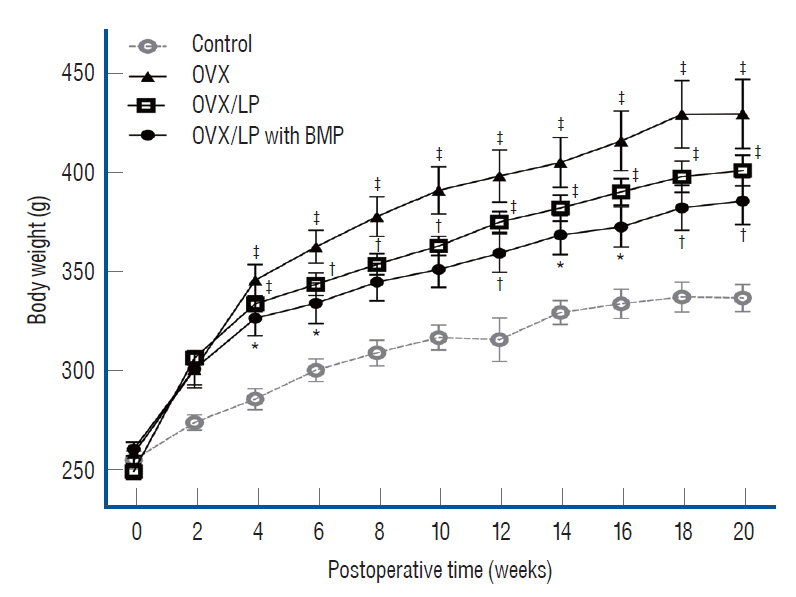
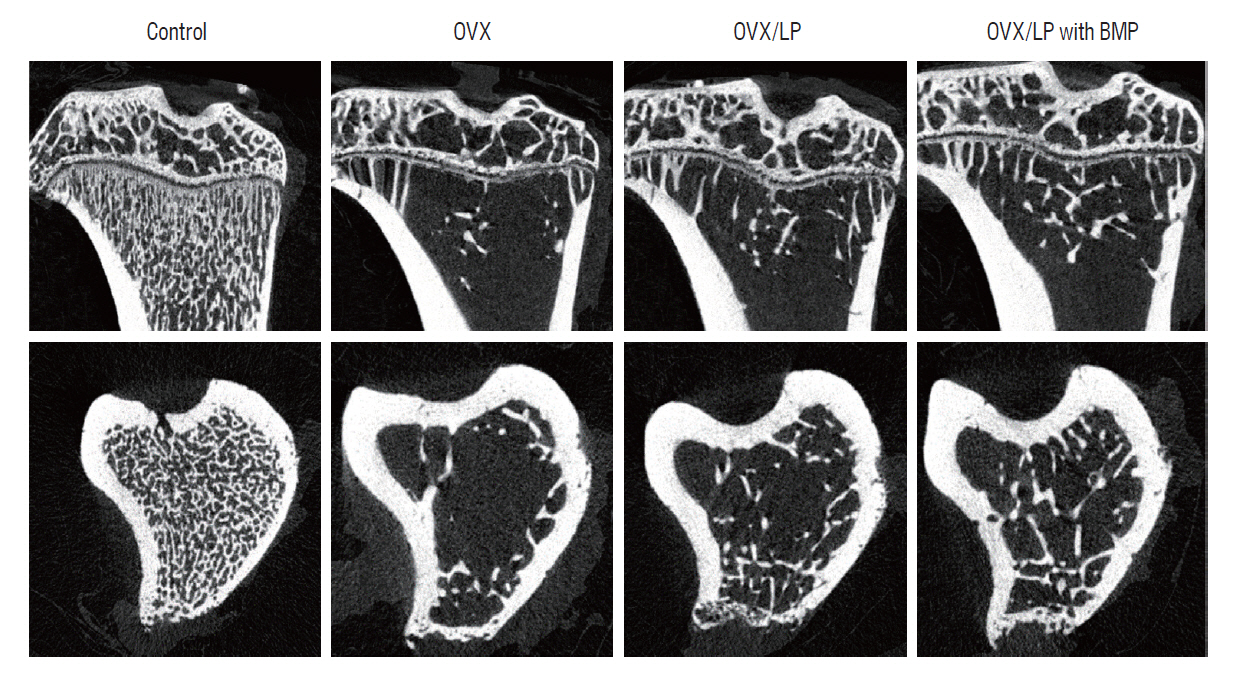
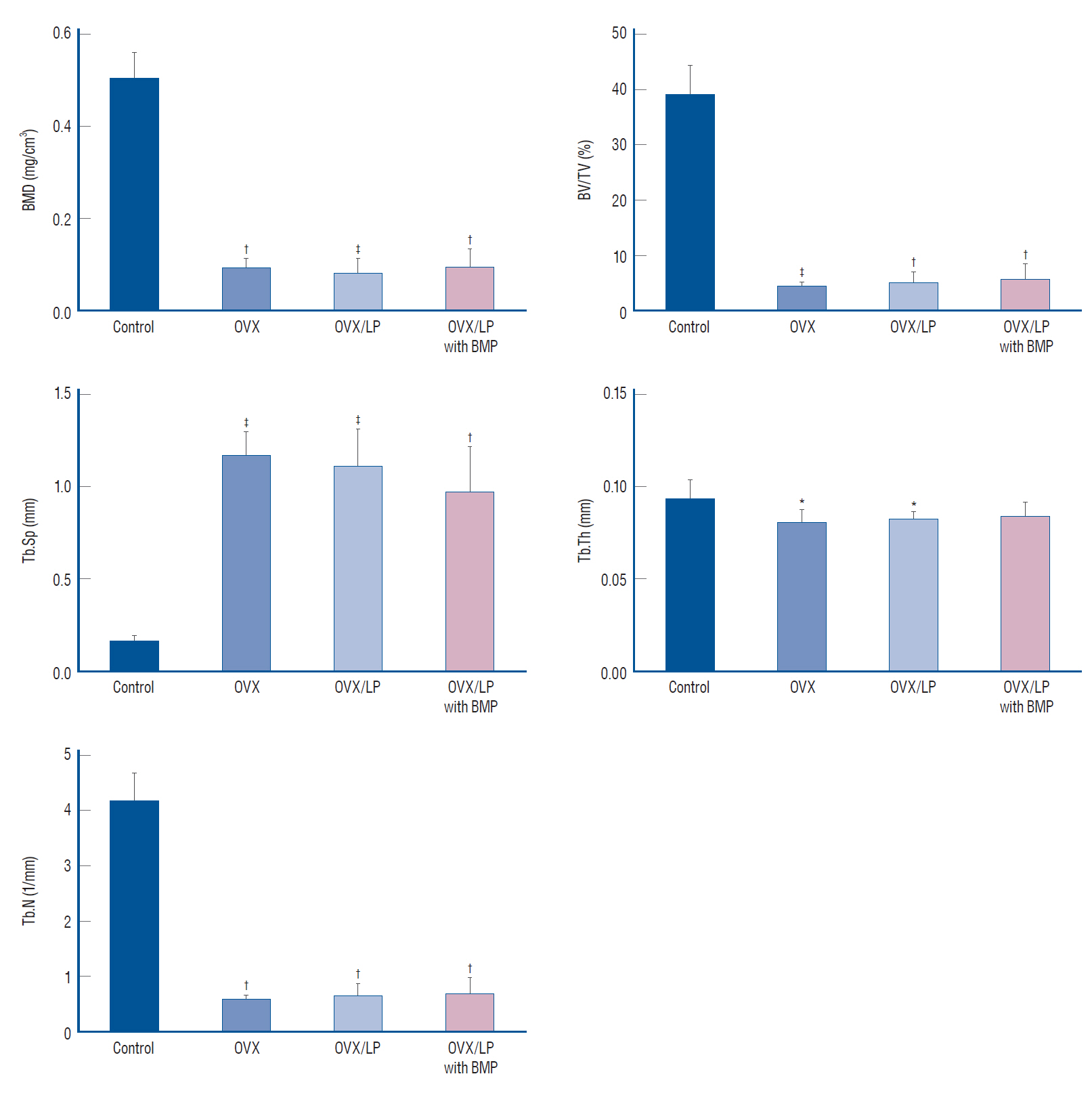
: Twenty-eight female Wistar rats (250–300 g, 12 weeks old) were divided into four groups : the sham (control), the ovariectomy (OVX)-induced osteoporosis group (OVX), the OVX and LP (OVX/LP), OVX and genetically modified BMP-2-expressing LP (OVX/LP with BMP) groups. The three groups underwent bilateral OVX and two of these groups were administered two different types of LP via oral gavage daily. At 16 weeks post-OVX, blood was collected from the heart and the bilateral tibiae were extracted and were scanned by ex-vivo micro-computed tomography and stained with hematoxylin-and-eosin (H&E) and Masson’s trichrome stain for pathological assessment. The serum levels of osteocalcin (OC), rat C-telopeptide of type I collagen (CTX-I), BMP-2, and receptor activator of nuclear factor-ĸB ligand (RANKL) were measured.
Results
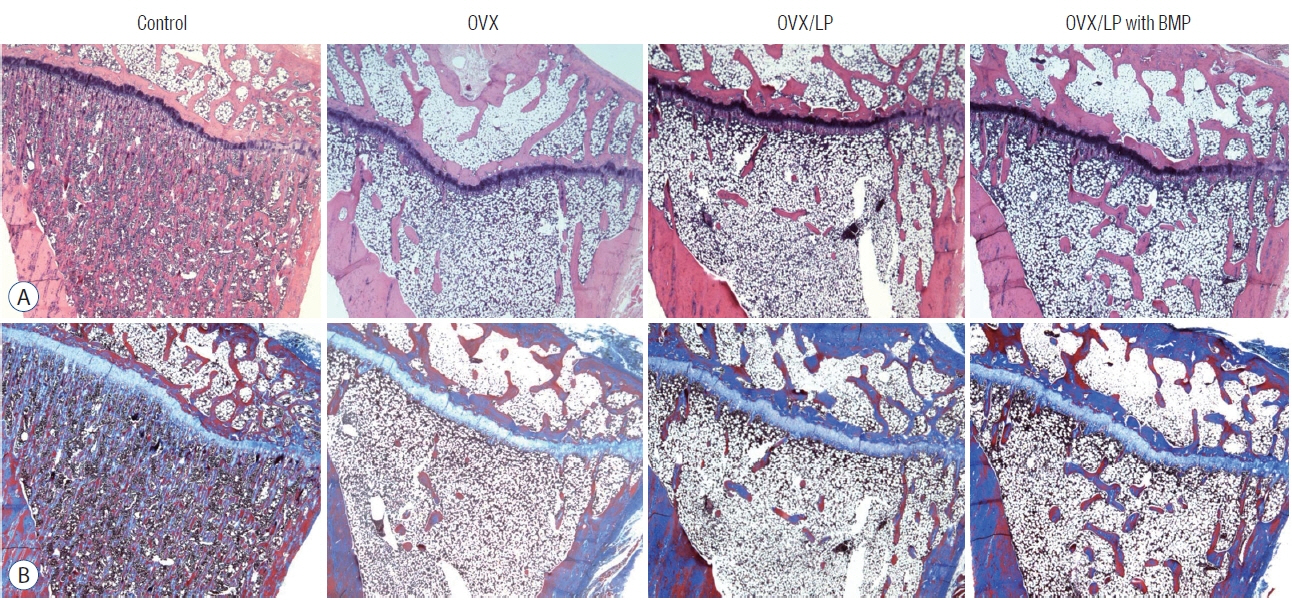
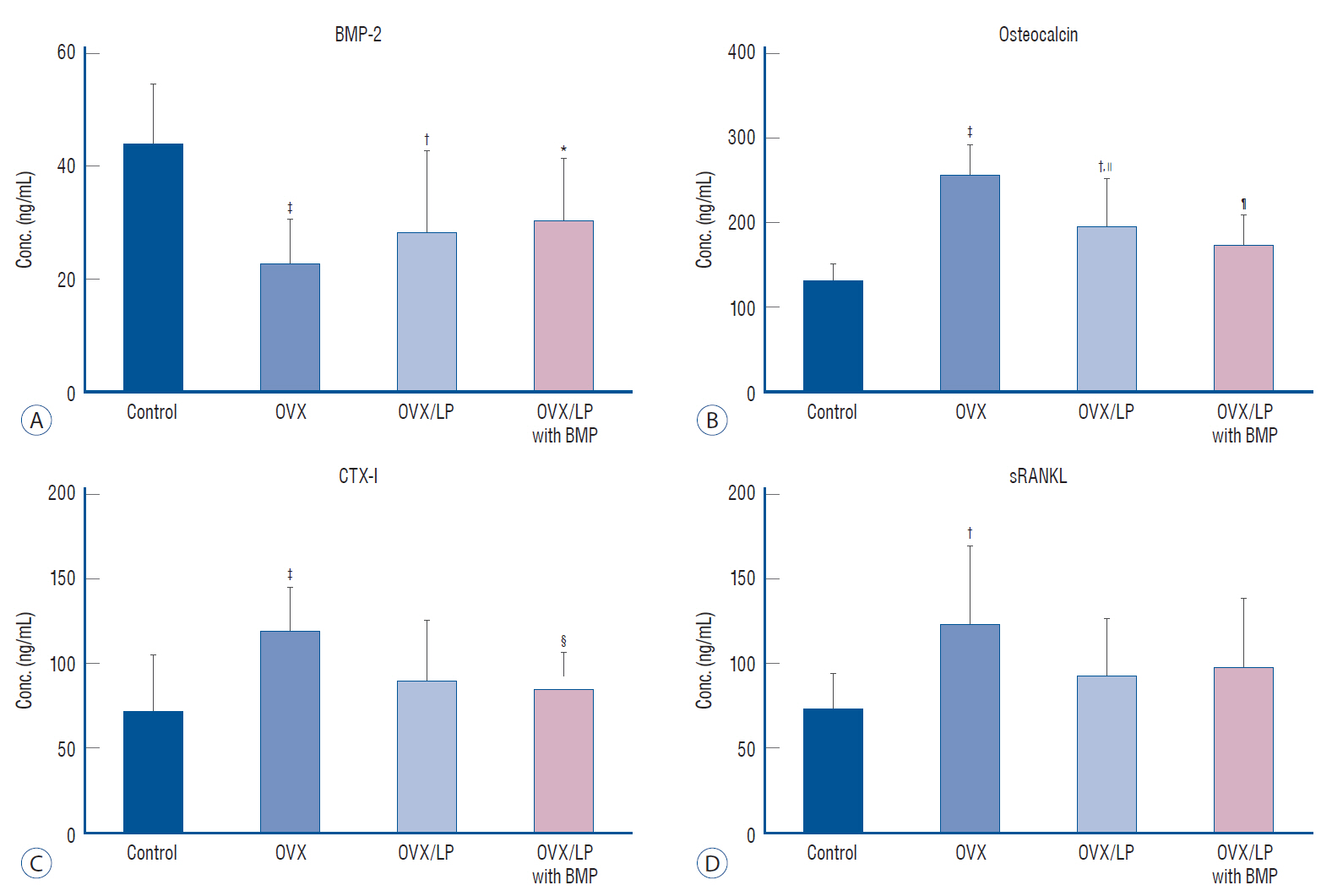
: The 3D-micro-computed tomography images showed that the trabecular structure in the OVX/LP with BMP group was maintained compared with OVX and OVX/LP groups. No significant differences were detected in trabecular thickness (Tb.Th) between control and OVX/LP with BMP groups (p>0.05). Furthermore, a tendency toward increased BMD, trabecular bone volume, Tb.Th, and trabecular number and decreased trabecular separation was found in rats in the OVX/LP with BMP groups when compared with the OVX and OVX/LP groups (p>0.05). The H&E and Masson’s trichrome stained sections showed a thicker trabecular bone in the OVX/LP with BMP group compared with the OVX and OVX/LP groups. There was no difference in serum levels of OC, CTX and RANKL control and OVX/LP with BMP groups (p>0.05). In contrast, significant differences were found in OC and CTX-1 levels between the OVX and OVX/LP with BMP groups (p<0.05).
Conclusion
: Our results showed that the expression of genetically modified BMP-2 showed inhibition effect for bone loss in a rat model of osteoporosis.
Figure
Reference
-
References
1. Abdul-Majeed S, Mohamed N, Soelaiman IN. Effects of tocotrienol and lovastatin combination on osteoblast and osteoclast activity in estrogen-deficient osteoporosis. Evid Based Complement Alternat Med. 2012:960742. 2012.
Article2. Astrand P, Ahlqvist J, Gunne J, Nilson H. Implant treatment of patients with edentulous jaws: a 20-year follow-up. Clin Implant Dent Relat Res. 10:207–217. 2008.3. Chilton SN, Burton JP, Reid G. Inclusion of fermented foods in food guides around the world. Nutrients. 7:390–404. 2015.
Article4. Christiansen C. Prevention and treatment of osteoporosis: a review of current modalities. Bone 13 Suppl. 1:S35–S39. 1992.
Article5. de Moreno de LeBlanc A, Del Carmen S, Chatel JM, Miyoshi A, Azevedo V, Langella P, et al. Current review of genetically modified lactic acid bacteria for the prevention and treatment of colitis using murine models. Gastroenterol Res Pract. 2015:146972. 2015.
Article6. de Vrese M, Schrezenmeir J. Probiotics, prebiotics, and synbiotics. Adv Biochem Eng Biotechnol. 111:1–66. 2008.
Article7. Eom JE, Moon SK, Moon GS. Heterologous production of pediocin PA-1 in Lactobacillus reuteri. J Microbiol Biotechnol. 20:1215–1218. 2010.
Article8. Friedenstein AJ. Precursor cells of mechanocytes. Int Rev Cytol. 47:327–359. 1976.
Article9. Howarth GS, Wang H. Role of endogenous microbiota, probiotics and their biological products in human health. Nutrients. 5:58–81. 2013.
Article10. Ivaska KK, Hentunen TA, Vääräniemi J, Ylipahkala H, Pettersson K, Väänänen HK. Release of intact and fragmented osteocalcin molecules from bone matrix during bone resorption in vitro. J Biol Chem. 279:18361–18369. 2004.
Article11. Jung UW, Choi SY, Pang EK, Kim CS, Choi SH, Cho KS. The effect of varying the particle size of beta tricalcium phosphate carrier of recombinant human bone morphogenetic protein-4 on bone formation in rat calvarial defects. J Periodontol. 77:765–772. 2006.
Article12. Kailasapathy K, Chin J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol Cell Biol. 78:80–88. 2000.
Article13. Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 15:175–191. 1991.
Article14. Kanakaris NK, Petsatodis G, Tagil M, Giannoudis PV. Is there a role for bone morphogenetic proteins in osteoporotic fractures? Injury. 40 Suppl 3:S21–S26. 2009.
Article15. Khosla S, Westendorf JJ, Oursler MJ. Building bone to reverse osteoporosis and repair fractures. J Clin Invest. 118:421–428. 2008.
Article16. Kimoto-Nira H, Suzuki C, Kobayashi M, Sasaki K, Kurisaki J, Mizumachi K. Anti-ageing effect of a lactococcal strain: analysis using senescence-accelerated mice. Br J Nutr. 98:1178–1186. 2007.
Article17. LeBlanc JG, Aubry C, Cortes-Perez NG, de Moreno de LeBlanc A, Vergnolle N, Langella P, et al. Mucosal targeting of therapeutic molecules using genetically modified lactic acid bacteria: an update. FEMS Microbiol Lett. 344:1–9. 2013.
Article18. Legrand E, Chappard D, Pascaretti C, Duquenne M, Krebs S, Rohmer V, et al. Trabecular bone microarchitecture, bone mineral density, and vertebral fractures in male osteoporosis. J Bone Miner Res. 15:13–19. 2000.
Article19. McCabe LR, Irwin R, Schaefer L, Britton RA. Probiotic use decreases intestinal inflammation and increases bone density in healthy male but not female mice. J Cell Physiol. 228:1793–1798. 2013.
Article20. Moon GS, Lee YD, Kim WJ. Screening of a novel lactobacilli replicon from plasmids of Lactobacillus reuteri KCTC 3678. Food Sci Biotechnol. 17:438–441. 2008.21. Moon GS, Pyun YR, Park MS, Ji GE, Kim WJ. Secretion of recombinant pediocin PA-1 by Bifidobacterium longum, using the signal sequence for bifidobacterial alpha-amylase. Appl Environ Microbiol. 71:5630–5632. 2005.
Article22. Moslehi-Jenabian S, Pedersen LL, Jespersen L. Beneficial effects of probiotic and food borne yeasts on human health. Nutrients. 2:449–473. 2010.
Article23. Mutuş R, Kocabagli N, Alp M, Acar N, Eren M, Gezen SS. The effect of dietary probiotic supplementation on tibial bone characteristics and strength in broilers. Poult Sci. 85:1621–1625. 2006.
Article24. Park SB, Lee YJ, Chung CK. Bone mineral density changes after ovariectomy in rats as an osteopenic model : stepwise description of double dorso-lateral approach. J Korean Neurosurg Soc. 48:309–312. 2010.
Article25. Parvaneh K, Ebrahimi M, Sabran MR, Karimi G, Hwei AN, Abdul-Majeed S, et al. Probiotics (Bifidobacterium longum) increase bone mass density and upregulate sparc and BMP-2 genes in rats with bone loss resulting from ovariectomy. Biomed Res Int. 2015:897639. 2015.26. Parvaneh K, Jamaluddin R, Karimi G, Erfani R. Effect of probiotics supplementation on bone mineral content and bone mass density. ScientificWorldJournal. 2014:595962. 2014.
Article27. Reid G, Sanders ME, Gaskins HR, Gibson GR, Mercenier A, Rastall R, et al. New scientific paradigms for probiotics and prebiotics. J Clin Gastroenterol. 37:105–118. 2003.
Article28. Romero Barco CM, Manrique Arija S, Rodríguez Pérez M. Biochemical markers in osteoporosis: usefulness in clinical practice. Reumatol Clin. 8:149–152. 2012.
Article29. Rosen HN, Moses AC, Garber J, Iloputaife ID, Ross DS, Lee SL, et al. Serum CTX: a new marker of bone resorption that shows treatment effect more often than other markers because of low coefficient of variability and large changes with bisphosphonate therapy. Calcif Tissue Int. 66:100–103. 2000.
Article30. Rubin CJ, Lindberg J, Fitzsimmons C, Savolainen P, Jensen P, Lundeberg J, et al. Differential gene expression in femoral bone from red junglefowl and domestic chicken, differing for bone phenotypic traits. BMC Genomics. 8:208. 2007.
Article31. Sakata T, Kojima T, Fujieda M, Miyakozawa M, Takahashi M, Ushida K. Probiotic preparations dose-dependently increase net production rates of organic acids and decrease that of ammonia by pig cecal bacteria in batch culture. Dig Dis Sci. 44:1485–1493. 1999.32. Scholz-Ahrens KE, Schrezenmeir J. Inulin and oligofructose and mineral metabolism: the evidence from animal trials. J Nutr. 137(11 Suppl):2513S–2523S. 2007.
Article33. Shim KS, Kim T, Ha H, Lee KJ, Cho CW, Kim HS, et al. Lactobacillus fermentation enhances the inhibitory effect of Hwangryun-haedok-tang in an ovariectomy-induced bone loss. BMC Complement Altern Med. 13:106. 2013.
Article34. Styrkarsdottir U, Cazier JB, Kong A, Rolfsson O, Larsen H, Bjarnadottir E, et al. Linkage of osteoporosis to chromosome 20p12 and association to BMP2. PLoS Biol. 1:E69. 2003.
Article35. Szulc P, Delmas PD. Biochemical markers of bone turnover: potential use in the investigation and management of postmenopausal osteoporosis. Osteoporos Int. 19:1683–1704. 2008.
Article36. Turner AS. Animal models of osteoporosis--necessity and limitations. Eur Cell Mater. 1:66–81. 2001.37. Urist MR. Bone morphogenetic protein: the molecularization of skeletal system development. J Bone Miner Res. 12:343–346. 1997.
Article38. Vitetta L, Briskey D, Alford H, Hall S, Coulson S. Probiotics, prebiotics and the gastrointestinal tract in health and disease. Inflammopharmacology. 22:135–154. 2014.
Article39. Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 29:2520–2526. 2014.
Article40. Yamaguchi K, Masuhara K, Yamasaki S, Nakai T, Fuji T. Cyclic therapy with etidronate has a therapeutic effect against local osteoporosis after cementless total hip arthroplasty. Bone. 33:144–149. 2003.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of composite of bone morphogenetic protein and plaster of paris on healing of bone defect in the rat tibia
- Immunogenicity of recombinant Lactobacillus plantarum NC8 expressing goose parvovirus VP2 gene in BALB/c mice
- The experimental study on the effect of pulsating electromagnetic fields in the osteoinduction induced by bone morphogenetic protein
- Adhesion Activity of Lactobacillus plantarum PM 008 Isolated from Kimchi on the Intestine of Mice
- Purification of porcine bone morphogenetic protein