J Bacteriol Virol.
2011 Jun;41(2):83-90. 10.4167/jbv.2011.41.2.83.
Adhesion Activity of Lactobacillus plantarum PM 008 Isolated from Kimchi on the Intestine of Mice
- Affiliations
-
- 1Department of Food and Nutrition, Kyung Hee University, Seoul, Korea. mjhan@khu.ac.kr
- 2Department of Life and Pharmaceutical Sciences, Kyung Hee University, Seoul, Korea. dhkim@khu.ac.kr
- 3Pulmuone Co., Seoul, Korea.
- KMID: 2168625
- DOI: http://doi.org/10.4167/jbv.2011.41.2.83
Abstract
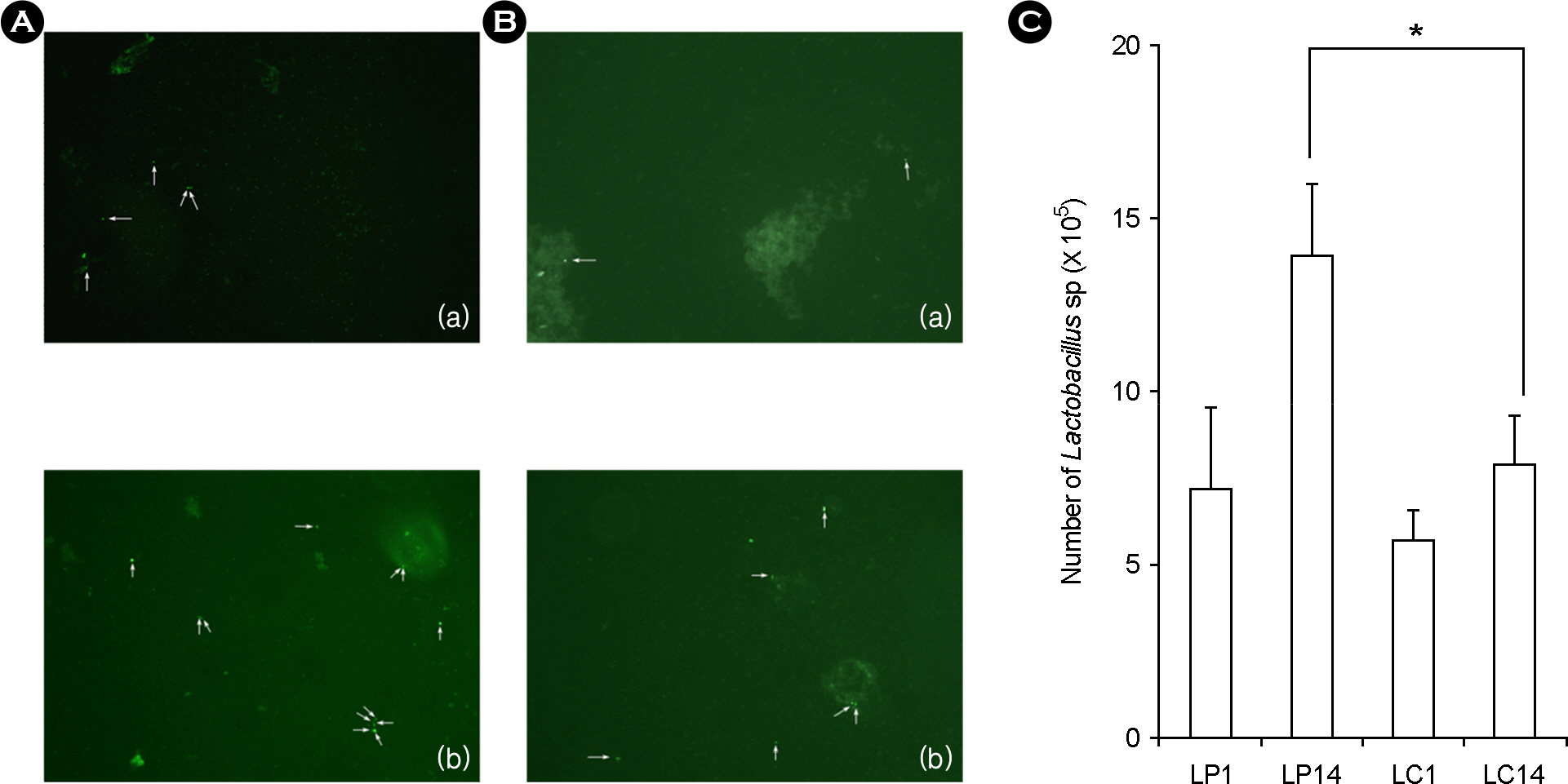
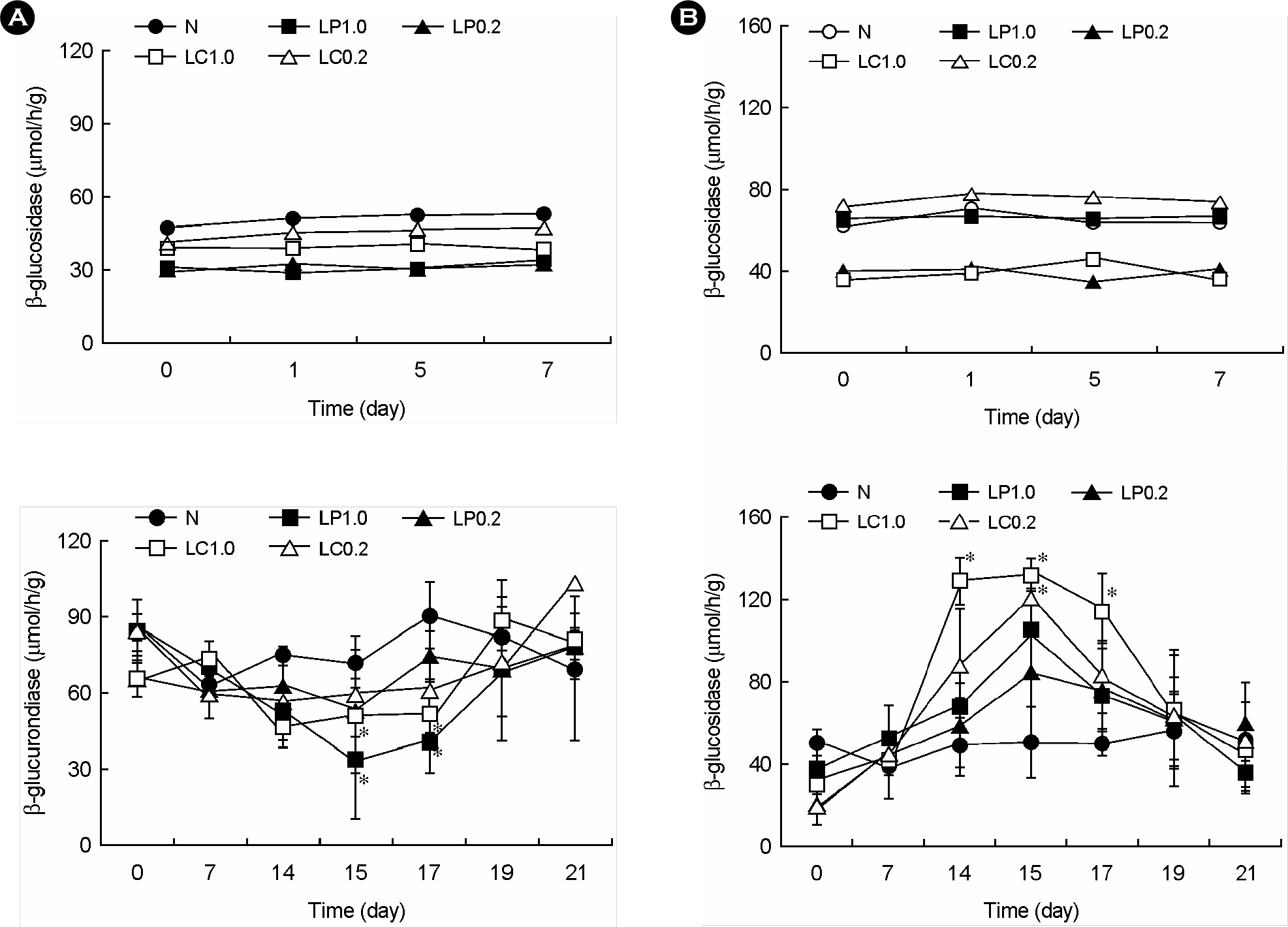
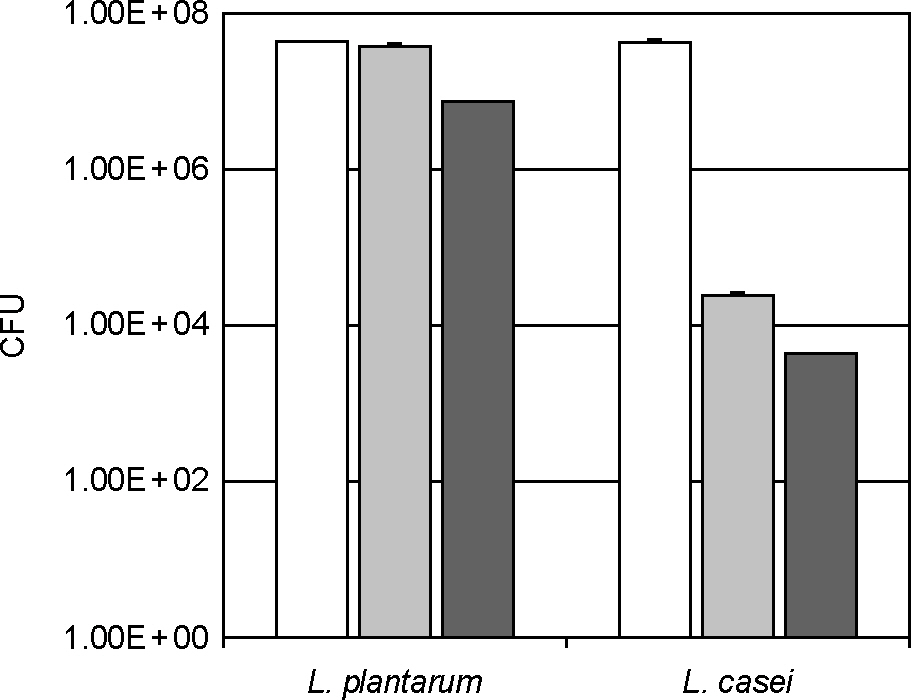
- Lactic acid bacteria (LAB), including L. plantarum isolated from Kimchi, are beneficial and safe microorganisms that improve disturbances of the indigenous microflora and the host's immune system. The adhesion abilities of Kimchi-derived L. plantarum PM008 and yogurt-derived L. casei were measured in vitro and in vivo. When L. plantarum or L. casei was incubated with Caco-2 cells, these Lactobacillus strains were potently attached. When these strains were orally administered to mice, the LABs were attached on the large intestine of mice. The attachment of L. plantarum on murine intestine or Caco-2 intestinal epithelial cell lines was more potent than that of L. casei, although numbers of LAB between their feces were not different. Treatment with either L. plantarum or L. casei for 14 days suppressed fecal beta-glucuronidase activity, although treatment for one day did not affect it. L. plantarum showed more potent inhibition than L. casei. In addition, L. plantarum and L. casei were stable to artificial gastric and intestinal juice. L. plantarum was more stable than L. casei. Based on these findings, the survival and adhesion effects of orally administered LAB strains in the intestine may increase numbers of LAB in intestine and express their biological activities.
MeSH Terms
Figure
Reference
-
1). Hill MJ., Drasar BS. The normal colonic bacterial flora. Gut. 1975. 16:318–23.
Article2). Simon GL., Gorbach SL. Intestinal flora in health and disease. Gastroenterology. 1984. 86:174–93.
Article3). Hattori M., Taylor TD. The human intestinal micro-biome: a new frontier of human biology. DNA Res. 2009. 16:1–12.
Article4). Chung KT., Fulk GE., Slein MW. Tryptophanase of fecal flora as a possible factor in the etiology of colon cancer. J Natl Cancer Inst. 1975. 54:1073–8.5). Ganguly NK., Kingham JG., Lloyd B., Lloyd RS., Price CP., Triger DR, et al. Acid hydrolases in monocytes from patients with inflammatory bowel disease, chronic liver disease, and rheumatoid arthritis. Lancet. 1978. 1:1073–5.
Article6). Rhodes JM., Gallimore R., Elias E., Allan RN., Kennedy JF. Faecal mucus degrading glycosidases in ulcerative colitis and Crohn's disease. Gut. 1985. 26:761–5.
Article7). Reddy BS., Weisburger JH., Wynder EL. Fecal bacterial beta-glucuronidase: control by diet. Science. 1974. 183:416–7.8). Collins MD., Gibson GR. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am J Clin Nutr. 1999. 69:1052S–1057S.
Article9). Campieri M., Gionchetti P. Probiotics in inflammatory bowel disease: New insight to pathogenesis or a possible therapeutic alternative? Gastroenterology. 1999. 116:1246–9.
Article10). Tabuchi M., Ozaki M., Tamura A., Yamada N., Ishida T., Hosoda M, et al. Antidiabetic effect of Lactobacillus GG in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem. 2003. 67:1421–4.11). Taranto MP., Medici M., Perdigon G., Ruiz Holgado AP., Valdez GF. Evidence for hypocholesterolemic effect of Lactobacillus reuteri in hypercholesterolemic mice. J Dairy Sci. 1998. 81:2336–40.12). Perdigon G., de Jorrat WEB., de Petrino SF., de Budeguer MV. Effect of oral administration of Lactobacillus casei on various biological functions of the host. Food Agric Immunol. 1991. 3:93–102.13). Daniel C., Poiret S., Goudercourt D., Dennin V., Leyer G., Pot B. Selecting lactic acid bacteria for their safety and functionality by use of a mouse colitis model. Appl Environ Microbiol. 2006. 72:5799–805.
Article14). Han W., Mercenier A., Ait-Belgnaoui A., Pavan S., Lamine F., van Swam II, et al. Improvement of an experimental colitis in rats by lactic acid bacteria producing super-oxide dismutase. Inflamm Bowel Dis. 2006. 12:1044–52.
Article15). Peran L., Sierra S., Comalada M., Lara-Villoslada F., Bailon E., Nieto A, et al. A comparative study of the preventative effects exerted by two probiotics, Lactobacillus reuteri and Lactobacillus fermentum, in the trinitrobenzenesulfonic acid model of rat colitis. Br J Nutr. 2007. 97:96–103.16). Mare L., Wolfaardt GM., Dicks LM. Adhesion of Lactobacillus plantarum 423 and Lactobacillus salivarius 241 to the intestinal tract of piglets, as recorded with fluorescent in situ hybridization (FISH), and production of plantaricin 423 by cells colonized to the ileum. J Appl Micribiol. 2006. 100:838–45.17). Sheiness D., Dix K., Watanabe S., Hillier SL. High levels of Gardnerella vaginalis detected with an oligonuceotide probe combined with elevated pH as a diagnostic indicator of bacterial vaginosis. J Clin Microbiol. 1992. 30:642–8.18). Amann RI., Binder BJ., Olson RJ., Chisholm SW., Devereux R., Stahl DA. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990. 56:1919–25.
Article19). Cho J., Lee D., Yang C., Jeon J., Kim J., Han H. Microbial population dynamics of kimchi, a fermented cabbage product. FEMS Microbiol Lett. 2006. 257:262–7.
Article20). Lee J., Hwang KT., Heo MS., Lee JH., Park KY. Resistance of Lactobacillus plantarum KCTC 3099 from Kimchi to oxidative stress. J Med Food. 2005. 8:299–304.21). Cho YR., Chang JY., Chang HC. Production of gamma-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. J Microbiol Biotechnol. 2007. 17:104–9.22). Parente E., Ciocia F., Ricciardi A., Zotta T., Felis GE., Torriani S. Diversity of stress tolerance in Lactobacillus plantarum, Lactobacillus pentosus and Lactobacillus paraplantarum: A multivariate screening study. Int J Food Microbiol. 2010. 144:270–9.23). Arena ME., Saguir FM., Manca de Nadra MC. Arginine dihydrolase pathway in Lactobacillus plantarum from orange. Int J Food Microbiol. 1999. 47:203–9.24). Siragusa S., De Angelis M., Di Cagno R., Rizzello CG., Coda R., Gobbetti M. Synthesis of gamma-amminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheeses. Appl Environ Microbiol. 2007. 73:7283–90.25). Maragkoudakis PA., Chingwaru W., Gradisnik L., Tsakalidou E., Cencic A. Lactic acid bacteria efficiently protect human and animal intestinal epithelial and immune cells from enteric virus infection. Int J Food Microbiol. 2010. ;141 Suppl. 1:S91–7.
Article26). Park HY., Bae EA., Han MJ., Choi EC., Kim DH. Inhibitory effects of Bifidobacterium spp. isolated from a healthy Korean on harmful enzymes of human intestinal microflora. Arch Pharm Res. 1998. 21:54–61.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characteristics of Vegetable Juice Fermented with Lactobacillus plantarum MKHA15 and Leuconostoc mesenteroids MKSR
- Immuno-enhancing Effects of Lactobacillus salivarius JWS 58 and Lactobacillus plantarum JWS 1354 isolated from duck
- Dietary effect of Lactobacillus plantarum CJLP55 isolated from kimchi on skin pH and its related biomarker levels in adult subjects
- Antifungal Activity of Lactic Acid Bacteria Isolated from Kimchi Against Aspergillus fumigatus
- Therapeutic effects of orally administered CJLP55 for atopic dermatitis via the regulation of immune response