J Korean Med Sci.
2022 Feb;37(6):e48. 10.3346/jkms.2022.37.e48.
Eltrombopag for Post-Transplant Poor Graft Function in East Asian Patients
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea
- 2Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2526036
- DOI: http://doi.org/10.3346/jkms.2022.37.e48
Abstract
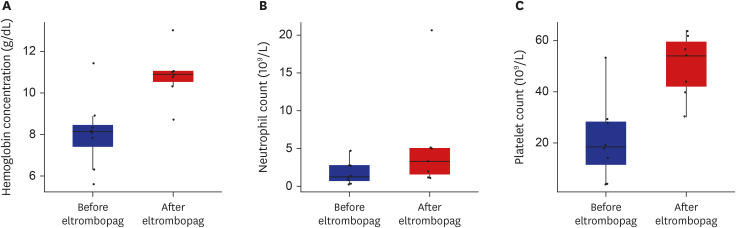
- Poor graft function (PGF) is a serious, potentially life-threatening complication of allogeneic hematopoietic stem cell transplantation. Eltrombopag has shown multilineage responses in patients with refractory severe aplastic anemia, supporting the idea that it may improve cytopenia in patients with PGF. This retrospective, single center analysis included 8 Korean patients receiving eltrombopag for PGF. Median interval between transplant and eltrombopag treatment was 73 days, and the median duration treatment was 3.5 weeks. With median maximum daily dose of 50 mg, the time to best response was 93 days. Median hemoglobin increased from 8.2 g/dL to 10.9 g/dL, platelet from 18.5 × 109 /L to 54 × 109 /L, and absolute neutrophil count from 1.25 × 109 /L to 3.32 × 109 /L. In conclusion, eltrombopag is a good option for PGF in Korean patients, even at a lower dose compared to western patients.
Keyword
Figure
Reference
-
1. Marotta S, Marano L, Ricci P, Cacace F, Frieri C, Simeone L, et al. Eltrombopag for post-transplant cytopenias due to poor graft function. Bone Marrow Transplant. 2019; 54(8):1346–1353. PMID: 30679824.2. Kong Y. Poor graft function after allogeneic hematopoietic stem cell transplantation-an old complication with new insights. Semin Hematol. 2019; 56(3):215–220. PMID: 31202433.3. Lee KH, Lee JH, Choi SJ, Lee JH, Kim S, Seol M, et al. Failure of trilineage blood cell reconstitution after initial neutrophil engraftment in patients undergoing allogeneic hematopoietic cell transplantation - frequency and outcomes. Bone Marrow Transplant. 2004; 33(7):729–734. PMID: 14755315.4. Stasia A, Ghiso A, Galaverna F, Raiola AM, Gualandi F, Luchetti S, et al. CD34 selected cells for the treatment of poor graft function after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2014; 20(9):1440–1443. PMID: 24862637.5. Liu X, Wu M, Peng Y, Chen X, Sun J, Huang F, et al. Improvement in poor graft function after allogeneic hematopoietic stem cell transplantation upon administration of mesenchymal stem cells from third-party donors: a pilot prospective study. Cell Transplant. 2014; 23(9):1087–1098. PMID: 23294601.6. Wong RS, Saleh MN, Khelif A, Salama A, Portella MS, Burgess P, et al. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: final results of the EXTEND study. Blood. 2017; 130(23):2527–2536. PMID: 29042367.7. Olnes MJ, Scheinberg P, Calvo KR, Desmond R, Tang Y, Dumitriu B, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012; 367(1):11–19. PMID: 22762314.8. Mahat U, Rotz SJ, Hanna R. Use of thrombopoietin receptor agonists in prolonged thrombocytopenia after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2020; 26(3):e65–e73. PMID: 31830528.9. Bento L, Bastida JM, García-Cadenas I, García-Torres E, Rivera D, Bosch-Vilaseca A, et al. Thrombopoietin receptor agonists for severe thrombocytopenia after allogeneic stem cell transplantation: experience of the Spanish Group of Hematopoietic Stem Cell Transplant. Biol Blood Marrow Transplant. 2019; 25(9):1825–1831. PMID: 31152794.10. Tang C, Chen F, Kong D, Ma Q, Dai H, Yin J, et al. Successful treatment of secondary poor graft function post allogeneic hematopoietic stem cell transplantation with eltrombopag. J Hematol Oncol. 2018; 11(1):103. PMID: 30115080.11. Halahleh K, Gale RP, Da’na W, Ma’koseh M, Saadeh S, Alan W, et al. Therapy of posttransplant poor graft function with eltrombopag. Bone Marrow Transplant. 2021; 56(1):4–6. PMID: 32572137.12. Kim YK, Lee SS, Jeong SH, Ahn JS, Yang DH, Lee JJ, et al. Efficacy and safety of eltrombopag in adult refractory immune thrombocytopenia. Blood Res. 2015; 50(1):19–25. PMID: 25830126.13. Erickson-Miller CL, Delorme E, Tian SS, Hopson CB, Landis AJ, Valoret EI, et al. Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist. Stem Cells. 2009; 27(2):424–430. PMID: 19038790.14. O’Neill A, Chin D, Tan D, Abdul Majeed AB, Nakamura-Ishizu A, Suda T. Thrombopoietin maintains cell numbers of hematopoietic stem and progenitor cells with megakaryopoietic potential. Haematologica. 2021; 106(7):1883–1891. PMID: 32527954.15. Shida Y, Takahashi N, Nohda S, Hirama T. Pharmacokinetics and pharmacodynamics of eltrombopag in healthy Japanese males. Jpn J Clin Pharmacol Ther. 2011; 42(1):11–20.16. Gibiansky E, Zhang J, Williams D, Wang Z, Ouellet D. Population pharmacokinetics of eltrombopag in healthy subjects and patients with chronic idiopathic thrombocytopenic purpura. J Clin Pharmacol. 2011; 51(6):842–856. PMID: 20663993.17. Tanaka T, Inamoto Y, Yamashita T, Fuji S, Okinaka K, Kurosawa S, et al. Eltrombopag for treatment of thrombocytopenia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2016; 22(5):919–924. PMID: 26785333.18. Rivera D, Bastida JM, Lopez-Corral L, Sanchez-Guijo F, Cabrero M, Martin A, et al. Usefulness of eltrombopag for treating thrombocytopenia after allogeneic stem cell transplantation. Bone Marrow Transplant. 2019; 54(5):757–761. PMID: 30356164.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical relevance of the Living Kidney Donor Profile Index in Asian kidney transplant recipients
- Impact of Graft Kidney Volume and Weight on Graft Function in Living Donor Kidney Transplantation
- Surgical Complications are Major Problems Concerning Overseas Kidney Transplantation in Comparison Study with Domestic Deceased Donor Kidney Transplantation
- The Trend and Prospect of Studies of East Asian Medical History in Korea
- The effect of a recipient’s body mass index to kidney transplantation outcomes: a retrospective cohort study at National Kidney and Transplant Institute