Cancer Res Treat.
2022 Jan;54(1):40-53. 10.4143/crt.2021.151.
Targeted Liquid Biopsy Using Irradiation to Facilitate the Release of Cell-Free DNA from a Spatially Aimed Tumor Tissue
- Affiliations
-
- 1Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Samsung Genome Institute, Samsung Medical Center, Seoul, Korea
- 3Department of Radiology and Center for Imaging Science, Sungkyunkwan University School of Medicine, Seoul, Korea
- 4GENINUS Inc., Seoul, Korea
- 5Department of Molecular Cell Biology, Sungkyunkwan University School of Medicine, Suwon, Korea
- KMID: 2524586
- DOI: http://doi.org/10.4143/crt.2021.151
Abstract
- Purpose
We investigated the feasibility of using an anatomically localized, target-enriched liquid biopsy (TLB) in mouse models of lung cancer.
Materials and Methods
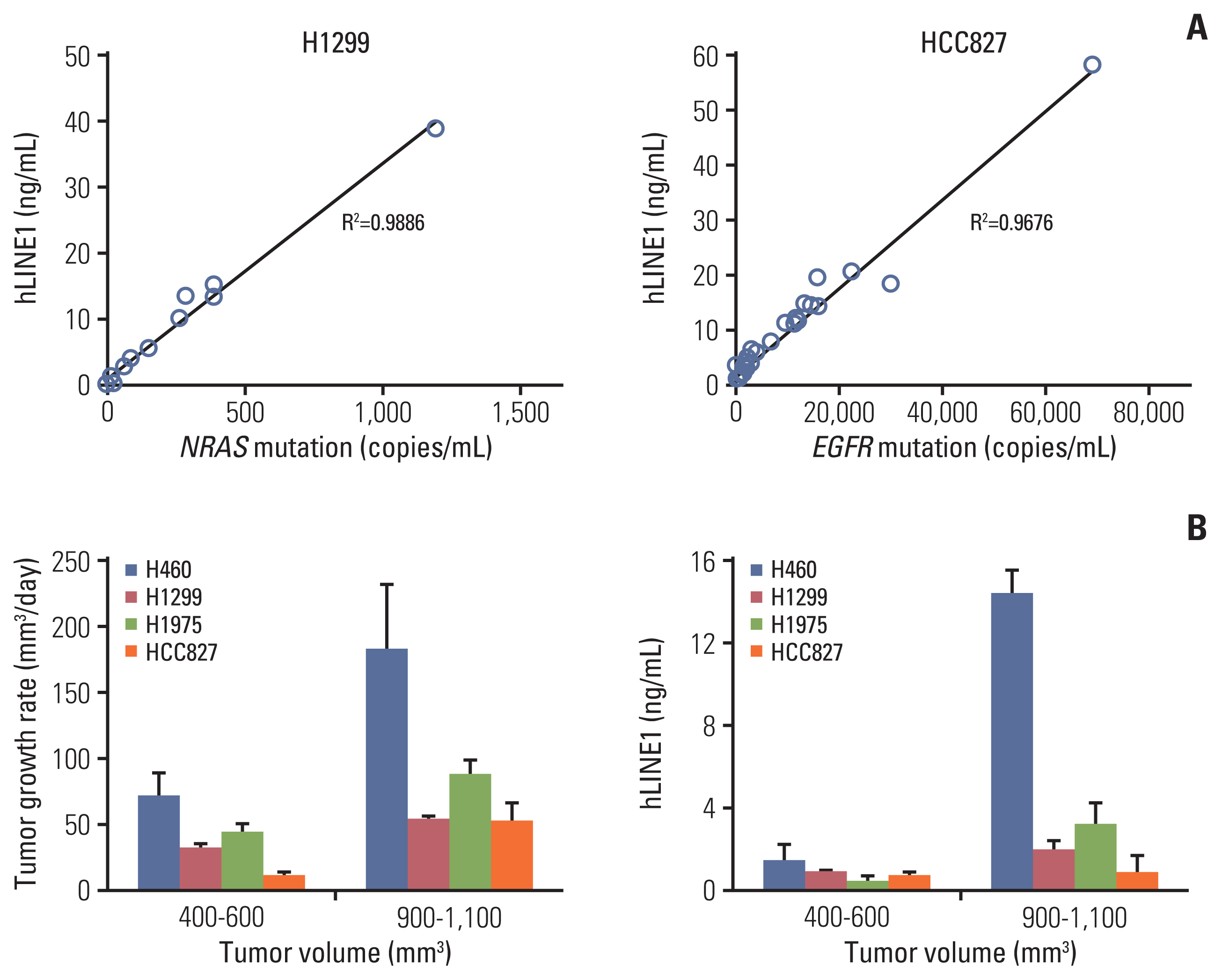
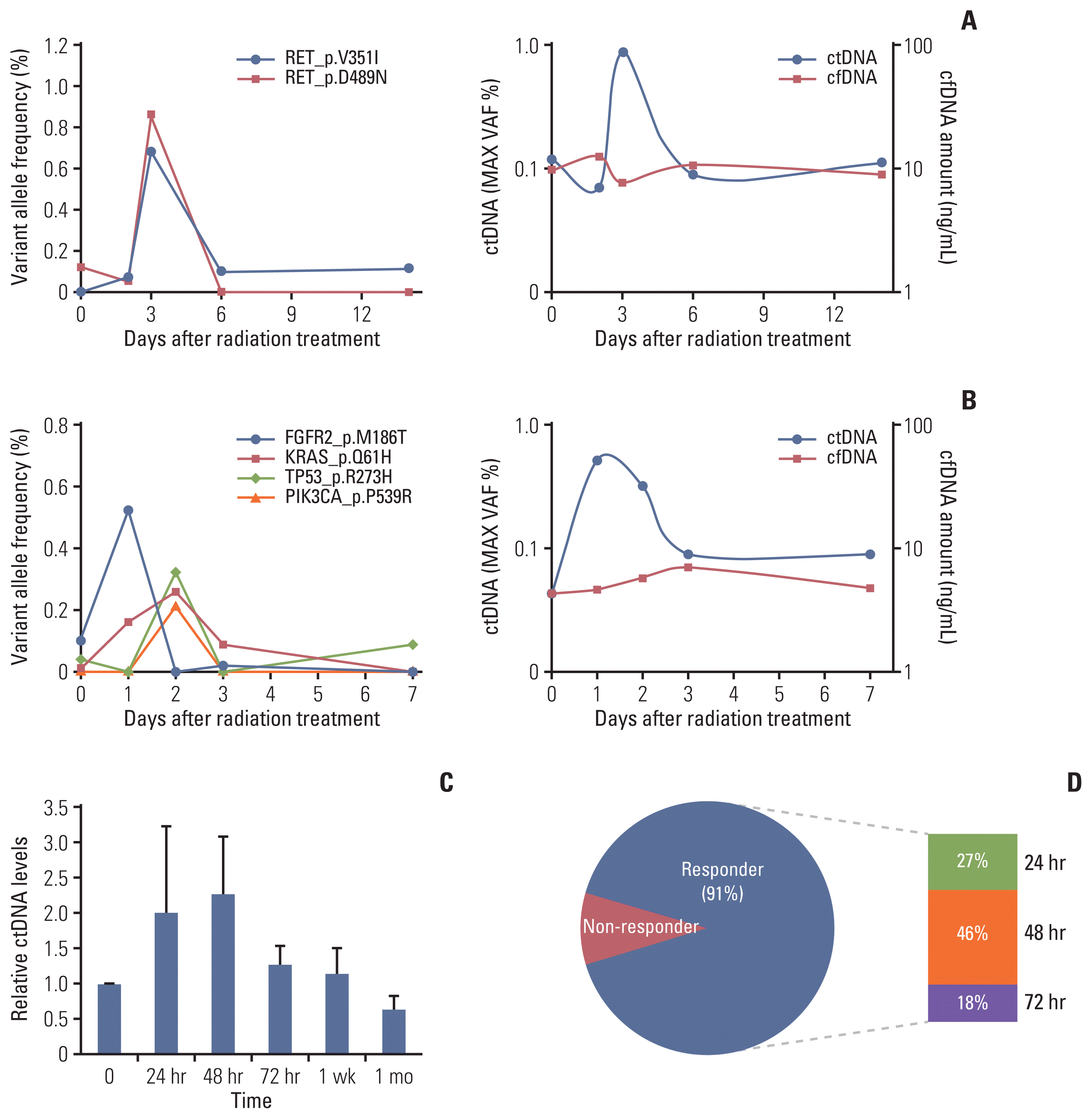
After irradiating xenograft mouse with human lung cancer cell lines, H1299 (NRAS proto-oncogene, GTPase [NRAS] Q61K) and HCC827 (epidermal growth factor receptor [EGFR] E746-750del), circulating (cell-free) tumor DNA (ctDNA) levels were monitored with quantitative polymerase chain reaction on human long interspersed nuclear element-1 and cell line-specific mutations. We checked dose-dependency at 6, 12, or 18 Gy to each tumor-bearing mouse leg using 6-MV photon beams. We also analyzed ctDNA of lung cancer patients by LiquidSCAN, a targeted deep sequencing to validated the clinical performances of TLB method.
Results
Irradiation could enhance the detection sensitivity of NRAS Q61K in the plasma sample of H1299-xenograft mouse to 4.5- fold. While cell-free DNA (cfDNA) level was not changed at 6 Gy, ctDNA level was increased upon irradiation. Using double-xenograft mouse with H1299 and HCC827, ctDNA polymerase chain reaction analysis with local irradiation in each region could specify mutation type matched to transplanted cell types, proposing an anatomically localized, TLB. Furthermore, when we performed targeted deep sequencing of cfDNA to monitor ctDNA level in 11 patients with lung cancer who underwent radiotherapy, the average ctDNA level was increased within a week after the start of radiotherapy.
Conclusion
TLB using irradiation could temporarily amplify ctDNA release in xenograft mouse and lung cancer patients, which enables us to develop theragnostic method for cancer patients with accurate ctDNA detection.
Keyword
Figure
Reference
-
References
1. Zheng D, Ye X, Zhang MZ, Sun Y, Wang JY, Ni J, et al. Plasma EGFR T790M ctDNA status is associated with clinical outcome in advanced NSCLC patients with acquired EGFR-TKI resistance. Sci Rep. 2016; 6:20913.
Article2. Moding EJ, Diehn M, Wakelee HA. Circulating tumor DNA testing in advanced non-small cell lung cancer. Lung Cancer. 2018; 119:42–7.
Article3. Newman AM, Bratman SV, To J, Wynne JF, Eclov NC, Modlin LA, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014; 20:548–54.
Article4. Kato K, Uchida J, Kukita Y, Kumagai T, Nishino K, Inoue T, et al. Numerical indices based on circulating tumor DNA for the evaluation of therapeutic response and disease progression in lung cancer patients. Sci Rep. 2016; 6:29093.
Article5. De Michino S, Aparnathi M, Rostami A, Lok BH, Bratman SV. The utility of liquid biopsies in radiation oncology. Int J Radiat Oncol Biol Phys. 2020; 107:873–86.
Article6. Rostami A, Bratman SV. Utilizing circulating tumour DNA in radiation oncology. Radiother Oncol. 2017; 124:357–64.
Article7. Baumann M, Krause M, Overgaard J, Debus J, Bentzen SM, Daartz J, et al. Radiation oncology in the era of precision medicine. Nat Rev Cancer. 2016; 16:234–49.
Article8. Chaudhuri AA, Binkley MS, Osmundson EC, Alizadeh AA, Diehn M. Predicting radiotherapy responses and treatment outcomes through analysis of circulating tumor DNA. Semin Radiat Oncol. 2015; 25:305–12.
Article9. Shin SH, Kim YJ, Lee D, Cho D, Ko YH, Cho J, et al. Analysis of circulating tumor DNA by targeted ultra-deep sequencing across various non-Hodgkin lymphoma subtypes. Leuk Lymphoma. 2019; 60:2237–46.
Article10. Wang X, Park J, Susztak K, Zhang NR, Li M. Bulk tissue cell type deconvolution with multi-subject single-cell expression reference. Nat Commun. 2019; 10:380.
Article11. Rago C, Huso DL, Diehl F, Karim B, Liu G, Papadopoulos N, et al. Serial assessment of human tumor burdens in mice by the analysis of circulating DNA. Cancer Res. 2007; 67:9364–70.
Article12. Arnold KM, Flynn NJ, Raben A, Romak L, Yu Y, Dicker AP, et al. The impact of radiation on the tumor microenvironment: effect of dose and fractionation schedules. Cancer Growth Metastasis. 2018; 11:1179064418761639.
Article13. Underwood JJ, Quadri RS, Kalva SP, Shah H, Sanjeevaiah AR, Beg MS, et al. Liquid biopsy for cancer: review and implications for the radiologist. Radiology. 2020; 294:5–17.14. Abbosh C, Birkbak NJ, Swanton C. Early stage NSCLC: challenges to implementing ctDNA-based screening and MRD detection. Nat Rev Clin Oncol. 2018; 15:577–86.15. Corcoran RB, Chabner BA. Application of cell-free DNA analysis to cancer treatment. N Engl J Med. 2018; 379:1754–65.
Article16. Rostami A, Lambie M, Yu CW, Stambolic V, Waldron JN, Bratman SV. Senescence, necrosis, and apoptosis govern circulating cell-free DNA release kinetics. Cell Rep. 2020; 31:107830.
Article17. Rakhit CP, Trigg RM, Le Quesne J, Kelly M, Shaw JA, Pritchard C, et al. Early detection of pre-malignant lesions in a KRAS(G12D)-driven mouse lung cancer model by monitoring circulating free DNA. Dis Model Mech. 2019; 12:dmm036863.
Article18. Walls GM, McConnell L, McAleese J, Murray P, Lynch TB, Savage K, et al. Early circulating tumour DNA kinetics measured by ultra-deep next-generation sequencing during radical radiotherapy for non-small cell lung cancer: a feasibility study. Radiat Oncol. 2020; 15:132.
Article19. Kato K, Uchida J, Kukita Y, Kumagai T, Nishino K, Inoue T, et al. Transient appearance of circulating tumor DNA associated with de novo treatment. Sci Rep. 2016; 6:38639.
Article20. Wan JC, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017; 17:223–38.
Article21. Gomez DR, Tang C, Zhang J, Blumenschein GR Jr, Hernandez M, Lee JJ, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019; 37:1558–65.
Article22. Iyengar P, Wardak Z, Gerber DE, Tumati V, Ahn C, Hughes RS, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2018; 4:e173501.23. Chaudhuri AA, Chabon JJ, Lovejoy AF, Newman AM, Stehr H, Azad TD, et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov. 2017; 7:1394–403.
Article24. Daly ME, Monjazeb AM, Kelly K. Clinical trials integrating immunotherapy and radiation for non-small-cell lung cancer. J Thorac Oncol. 2015; 10:1685–93.
Article25. Dolgin E. News feature: radiation redux. Proc Natl Acad Sci U S A. 2017; 114:6416–20.
Article26. Hendriks LE, Menis J, De Ruysscher DK, Reck M. Combination of immunotherapy and radiotherapy: the next magic step in the management of lung cancer? J Thorac Oncol. 2020; 15:166–9.27. Moding EJ, Liu Y, Nabet BY, Chabon JJ, Chaudhuri AA, Hui AB, et al. Circulating tumor DNA dynamics predict benefit from consolidation immunotherapy in locally advanced non-small-cell lung cancer. Nat Cancer. 2020; 1:176–83.
Article28. Lv J, Chen Y, Zhou G, Qi Z, Tan KR, Wang H, et al. Liquid biopsy tracking during sequential chemo-radiotherapy identifies distinct prognostic phenotypes in nasopharyngeal carcinoma. Nat Commun. 2019; 10:3941.
Article29. Chera BS, Kumar S, Beaty BT, Marron D, Jefferys S, Green R, et al. Rapid clearance profile of plasma circulating tumor HPV type 16 DNA during chemoradiotherapy correlates with disease control in HPV-associated oropharyngeal cancer. Clin Cancer Res. 2019; 25:4682–90.
Article30. Hietanen T, Pitkanen M, Kapanen M, Kellokumpu-Lehtinen PL. Effects of single and fractionated irradiation on natural killer cell populations: radiobiological characteristics of viability and cytotoxicity in vitro. Anticancer Res. 2015; 35:5193–200.31. Wu Q, Allouch A, Martins I, Modjtahedi N, Deutsch E, Perfettini JL. Macrophage biology plays a central role during ionizing radiation-elicited tumor response. Biomed J. 2017; 40:200–11.
Article32. Shi X, Shiao SL. The role of macrophage phenotype in regulating the response to radiation therapy. Transl Res. 2018; 191:64–80.
Article33. Choi JJ, Reich CF 3rd, Pisetsky DS. The role of macrophages in the in vitro generation of extracellular DNA from apoptotic and necrotic cells. Immunology. 2005; 115:55–62.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Application of Circulating Tumor DNA Analysis
- Role of Liquid Biopsies in Colorectal Cancer
- Circulating Tumor DNA in a Breast Cancer Patient's Plasma Represents Driver Alterations in the Tumor Tissue
- Liquid biopsy for early detection and therapeutic monitoring of hepatocellular carcinoma
- Detection of KRAS mutations in plasma cell-free DNA of colorectal cancer patients and comparison with cancer panel data for tissue samples of the same cancers