KOBIO, the First Web-based Korean Biologics Registry Operated With a Unified Platform Among Distinct Disease Entities
- Affiliations
-
- 1Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea
- 2Division of Rheumatology, Department of Internal Medicine, Bucheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Division of Rheumatology, Department of Internal Medicine, Korea University Ansan Hospital, Ansan, Korea
- 4Division of Rheumatology, Department of Internal Medicine, Soonchunhyang University Bucheon Hospital, Bucheon, Korea
- 5Division of Rheumatology, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 6Division of Rheumatology, Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, Korea
- 7Department of Rheumatology, Hanyang University Hospital for Rheumatic Diseases, Seoul, Korea
- 8Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 9Department of Internal Medicine, Wonkwang University Hospital, Iksan, Korea
- 10Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea
- 11Division of Rheumatology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 12Department of Rheumatology, Chonnam National University Hospital, Gwangju, Korea
- 13Division of Rheumatology, Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 14Department of Rheumatology, Ajou University School of Medicine, Suwon, Korea
- 15Division of Rheumatology, Department of Internal Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea
- KMID: 2520430
- DOI: http://doi.org/10.4078/jrd.2021.28.4.176
Abstract
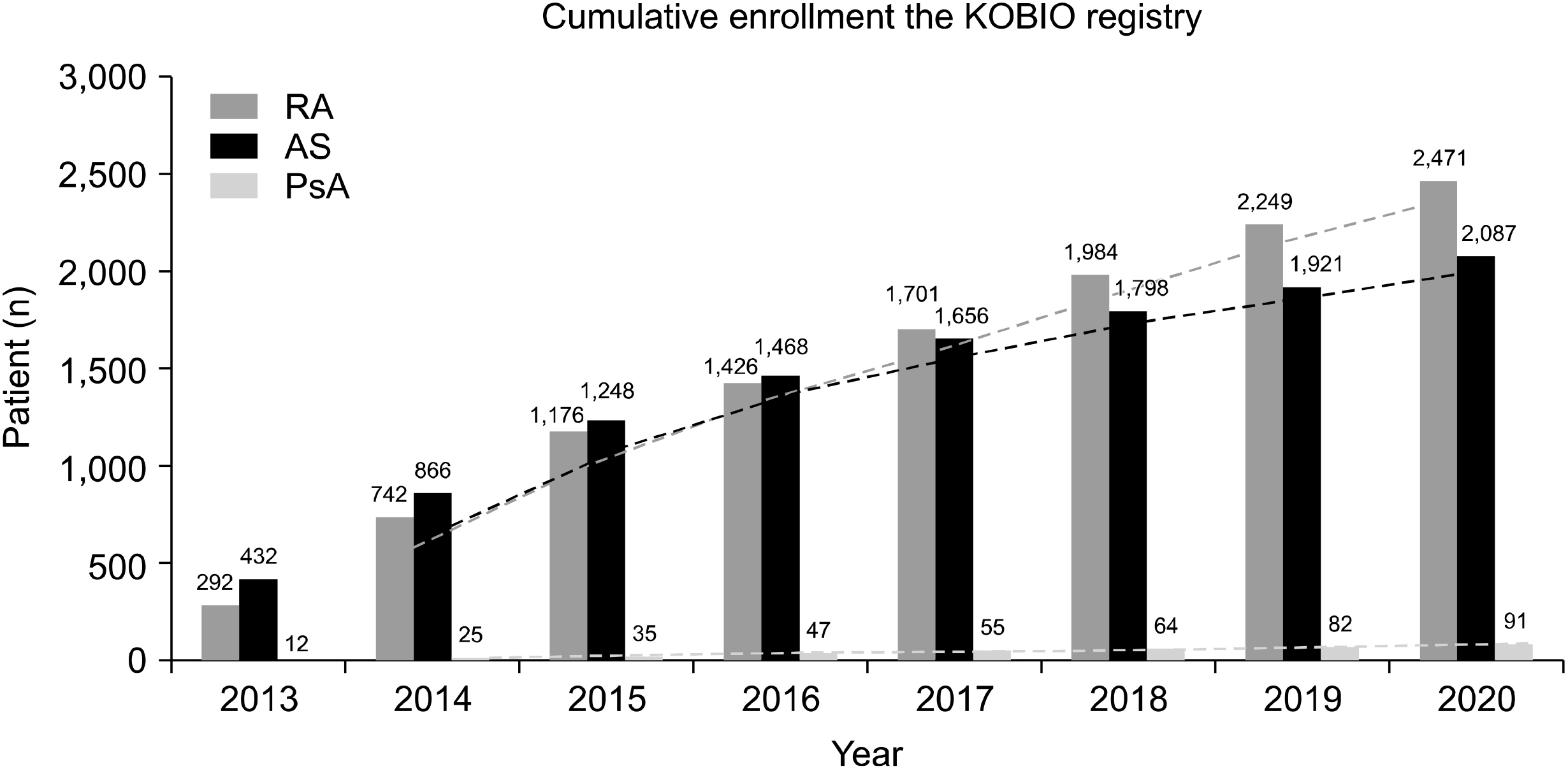
- The KOrean College of Rheumatology BIOlogics and targeted therapy (KOBIO) registry is a nationwide observational cohort that captures detailed data on exposure of patients to biologic and targeted synthetic disease-modifying anti-rheumatic drugs (DMARDs). This registry was launched in December 2012 with an aim to prospectively investigate clinical manifestations and outcomes of patients with rheumatoid arthritis (RA), ankylosing spondylitis, and psoriatic arthritis who initiated a biologic or targeted synthetic DMARD or switched to another. Demographic data, disease activity, current treatment, adverse events, terms based on Medical Dictionary for Regulatory Activities, and so on are registered for patients who are then followed up annually in a web-based unified platform. The KOBIO registry also recruits and collects data of patients with RA on conventional DMARDs for comparison. As of today, more than 5,500 patients were enrolled from 47 academic and community Rheumatology centers across Korea. The KOBIO registry has evolved to become a powerful database for clinical research to improve clinical outcomes and quality of treatment.
Keyword
Figure
Cited by 5 articles
-
The Korean College of Rheumatology: 40 Years of Public Health Influence
Jisoo Lee, Yoon-Kyoung Sung, Myeung-Su Lee, Han Joo Baek
J Rheum Dis. 2022;29(2):75-78. doi: 10.4078/jrd.2022.29.2.75.Clinical Characteristics of Patients With Psoriatic Spondylitis Versus Those With Ankylosing Spondylitis: Features at Baseline Before Biologic Therapy
Hyoun-Ah Kim, Eunyoung Lee, So Young Park, Shin-Seok Lee, Kichul Shin
J Korean Med Sci. 2022;37(33):e253. doi: 10.3346/jkms.2022.37.e253.The Epidemiology and Treatment of Ankylosing Spondylitis in Korea
Seong-Ryul Kwon, Tae-Hwan Kim, Tae-Jong Kim, Won Park, Seung Cheol Shim
J Rheum Dis. 2022;29(4):193-199. doi: 10.4078/jrd.22.0023.Implications of Persistent Pain in Patients With Rheumatoid Arthritis Despite Remission Status: Data From the KOBIO Registry
Hyoun-Ah Kim, So Young Park, Kichul Shin
J Rheum Dis. 2022;29(4):215-222. doi: 10.4078/jrd.22.0005.Pregnancy Outcomes Associated With Biologic Agent Exposure in Patients With Several Rheumatic Diseases and Inflammatory Bowel Diseases
Soo Min Ahn, Young Bin Joo, Yun Jin Kim, So-Young Bang, Hye-Soon Lee
J Korean Med Sci. 2023;38(22):e172. doi: 10.3346/jkms.2023.38.e172.
Reference
-
1. Baumfeld Andre E, Reynolds R, Caubel P, Azoulay L, Dreyer NA. 2020; Trial designs using real-world data: the changing landscape of the regulatory approval process. Pharmacoepidemiol Drug Saf. 29:1201–12. DOI: 10.1002/pds.4932. PMID: 31823482. PMCID: PMC7687110.
Article2. Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, et al. 2016; Real-world evidence - what is it and what can it tell us? N Engl J Med. 375:2293–7. DOI: 10.1056/NEJMsb1609216. PMID: 27959688.
Article3. Booth CM, Tannock IF. 2014; Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. Br J Cancer. 110:551–5. DOI: 10.1038/bjc.2013.725. PMID: 24495873. PMCID: PMC3915111.
Article4. Gliklich RE, Dreyer NA, Leavy MB. Gliklich RE, Dreyer NA, Leavy MB, editors. 2014. Patient registries. Registries for evaluating patient outcomes: a user's guide. 3rd ed. Agency for Healthcare Research and Quality (US);Rockville (MD):5. Nikiphorou E, Buch MH, Hyrich KL. 2017; Biologics registers in RA: methodological aspects, current role and future applications. Nat Rev Rheumatol. 13:503–10. DOI: 10.1038/nrrheum.2017.81. PMID: 28569267.
Article6. Mercer LK, Regierer AC, Mariette X, Dixon WG, Baecklund E, Hellgren K, et al. 2017; Spectrum of lymphomas across different drug treatment groups in rheumatoid arthritis: a European registries collaborative project. Ann Rheum Dis. 76:2025–30. DOI: 10.1136/annrheumdis-2017-211623. PMID: 28822981. PMCID: PMC5705847.
Article7. Mercer LK, Askling J, Raaschou P, Dixon WG, Dreyer L, Hetland ML, et al. 2017; Risk of invasive melanoma in patients with rheumatoid arthritis treated with biologics: results from a collaborative project of 11 European biologic registers. Ann Rheum Dis. 76:386–91. DOI: 10.1136/annrheumdis-2016-209285. PMID: 27307502. PMCID: PMC5284347.
Article8. Askling J, Fored CM, Brandt L, Baecklund E, Bertilsson L, Feltelius N, et al. 2007; Time-dependent increase in risk of hospitalisation with infection among Swedish RA patients treated with TNF antagonists. Ann Rheum Dis. 66:1339–44. DOI: 10.1136/ard.2006.062760. PMID: 17261532. PMCID: PMC1994293.
Article9. Hetland ML, Christensen IJ, Tarp U, Dreyer L, Hansen A, Hansen IT, et al. 2010; Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 62:22–32. DOI: 10.1002/art.27227. PMID: 20039405.
Article10. Iannone F, Gremese E, Atzeni F, Biasi D, Botsios C, Cipriani P, et al. 2012; Longterm retention of tumor necrosis factor-α inhibitor therapy in a large Italian cohort of patients with rheumatoid arthritis from the GISEA registry: an appraisal of predictors. J Rheumatol. 39:1179–84. DOI: 10.3899/jrheum.111125. PMID: 22467933.
Article11. Brown EG. 2004; Using MedDRA: implications for risk management. Drug Saf. 27:591–602. DOI: 10.2165/00002018-200427080-00010. PMID: 15154830.12. MedDRA. Medical Dictionary for Regulatory Activities [Internet]. MedDRA;McLean (VA): Available from: http://www.meddra.org/. cited 2021 Mar.13. Ministry of Food and Drug Safety. 2019. Sep. 11. ICH distributes the Korean version of International Medical Terminology (MedDRA) [Internet]. Ministry of Food and Drug Safety;Cheongju: Available from: https://www.mfds.go.kr/brd/m_99/down.do?brd_id=ntc0021&seq=43693&data_tp=A&file_seq=1. cited 2021 Jun 4.14. MedDRA. 2020. Mar. Introductory Guide MedDRA version 23.0 [Internet]. MedDRA;McLean (VA): Available from: https://admin.new.meddra.org/sites/default/files/guidance/file/intguide_%2023_0_English.pdf. cited 2021 Jun 5.15. Zavada J, Dixon WG, Askling J. 2014; Launch of a checklist for reporting longitudinal observational drug studies in rheumatology: a EULAR extension of STROBE guidelines based on experience from biologics registries. Ann Rheum Dis. 73:628. DOI: 10.1136/annrheumdis-2013-204102. PMID: 24058015.
Article16. Zink A, Askling J, Dixon WG, Klareskog L, Silman AJ, Symmons DP. 2009; European biologicals registers: methodology, selected results and perspectives. Ann Rheum Dis. 68:1240–6. DOI: 10.1136/ard.2008.091926. PMID: 18647854.
Article17. Canhão H, Faustino A, Martins F, Fonseca JE. 2011; Reuma.pt - the rheumatic diseases Portuguese register. Acta Reumatol Port. 36:45–56. PMID: 21483280.18. Carmona L, de la Vega M, Ranza R, Casado G, Titton DC, Descalzo MÁ, et al. 2014; BIOBADASER, BIOBADAMERICA, and BIOBADADERM: safety registers sharing commonalities across diseases and countries. Clin Exp Rheumatol. 32(5 Suppl 85):S-163-7. PMID: 25365109.19. Kvien TK, Heiberg , Lie E, Kaufmann C, Mikkelsen K, Nordvåg BY, et al. 2005; A Norwegian DMARD register: prescriptions of DMARDs and biological agents to patients with inflammatory rheumatic diseases. Clin Exp Rheumatol. 23(5 Suppl 39):S188–94. PMID: 16273806.20. Silman A, Symmons D, Scott DG, Griffiths I. 2003; British Society for Rheumatology Biologics Register. Ann Rheum Dis. 62(Suppl 2):ii28–9. DOI: 10.1136/ard.62.suppl_2.ii28. PMID: 14532144. PMCID: PMC1766752.
Article21. Zink A, Listing J, Kary S, Ramlau P, Stoyanova-Scholz M, Babinsky K, et al. 2005; Treatment continuation in patients receiving biological agents or conventional DMARD therapy. Ann Rheum Dis. 64:1274–9. DOI: 10.1136/ard.2004.031476. PMID: 15708884. PMCID: PMC1755655.
Article22. Hetland ML, Unkerskov J, Ravn T, Friis M, Tarp U, Andersen LS, et al. 2005; Routine database registration of biological therapy increases the reporting of adverse events twentyfold in clinical practice. First results from the Danish Database (DANBIO). Scand J Rheumatol. 34:40–4. DOI: 10.1080/03009740510017968. PMID: 15903024.
Article23. Lee SY. 2020. Oct. 13. KOBIO data analysis for World Arthritis Day [Internet]. The Medical Herald;Seoul: http://www.mediherald.com/news/articleView.html?idxno=61666. cited 2021 Jul 9.24. Kim SK, Choe JY, Lee SS, Shin K. 2017; Body mass index is related with the presence of syndesmophyte in axial spondyloarthritis: data from the Korean College of Rheumatology BIOlogics (KOBIO) registry. Mod Rheumatol. 27:855–61. DOI: 10.1080/14397595.2016.1265637. PMID: 27919202.
Article25. Kim SK, Kwak SG, Choe JY. 2020; Association between biologic disease modifying anti-rheumatic drugs and incident hypertension in patients with rheumatoid arthritis: results from prospective nationwide KOBIO Registry. Medicine (Baltimore). 99:e19415. DOI: 10.1097/MD.0000000000019415. PMID: 32118795. PMCID: PMC7478791.26. Kim SK, Choe JY, Kwak SG, Bae J, Park SH, Lee H. 2018; Effect of tumour necrosis factor-alpha inhibitors on renal function in patients with rheumatoid arthritis from the KOBIO registry from 2012 to 2016. Clin Exp Rheumatol. 36:1022–30. PMID: 29652655.27. Min HK, Kim HR, Lee SH, Shin K, Kim HA, Park SH, et al. 2021; Four-year follow-up of atherogenicity in rheumatoid arthritis patients: from the nationwide Korean College of Rheumatology Biologics Registry. Clin Rheumatol. 40:3105–13. DOI: 10.1007/s10067-021-05613-x. PMID: 33576925.
Article28. Kim Y, Park S, Kim HS. 2018; The effect of extra-articular manifestations on tumor necrosis factor-α inhibitor treatment duration in patients with ankylosing spondylitis: nationwide data from the Korean College of Rheumatology BIOlogics (KOBIO) registry. Clin Rheumatol. 37:3275–84. DOI: 10.1007/s10067-018-4290-0. PMID: 30251059.
Article29. Park DJ, Choi SJ, Shin K, Kim HA, Park YB, Kang SW, et al. 2017; Switching profiles in a population-based cohort of rheumatoid arthritis receiving biologic therapy: results from the KOBIO registry. Clin Rheumatol. 36:1013–22. DOI: 10.1007/s10067-017-3584-y. PMID: 28243760.
Article30. Ki Min H, Kim HR, Lee SH, Hong YS, Kim MY, Park SH, et al. 2021; Mar. 16. Retention rate and effectiveness of secukinumab vs TNF inhibitor in ankylosing spondylitis patients with prior TNF inhibitor exposure. Rheumatology (Oxford). [Epub]. DOI:10.1093/rheumatology/keab245. DOI: 10.1093/rheumatology/keab245. PMID: 33725088.
Article31. Jung SM, Lee SW, Song JJ, Park SH, Park YB. 2020; Dec. 14. Drug survival of biologic therapy in elderly patients with rheumatoid arthritis compared with nonelderly patients: results from the Korean College of Rheumatology Biologics registry. J Clin Rheumatol. [Epub]. DOI:10.1097/RHU.0000 000000001644. DOI: 10.1097/RHU.0000000000001644. PMID: 33337811.32. Jung SM, Kwok SK, Ju JH, Lee SW, Song JJ, Yoon CH, et al. 2018; Risk factors associated with inadequate control of disease activity in elderly patients with rheumatoid arthritis: results from a nationwide KOrean College of Rheumatology BIOlogics (KOBIO) registry. PLoS One. 13:e0205651. DOI: 10.1371/journal.pone.0205651. PMID: 30325962. PMCID: PMC6191131.
Article33. Koh JH, Lee SK, Kim J, Kim HA, Shin K, Min JK. 2021; Effectiveness and safety of biologic and targeted synthetic disease-modifying anti-rheumatic drugs in elderly patients with rheumatoid arthritis: real-world data from the KOBIO Registry. Clin Exp Rheumatol. 39:269–78. PMID: 32324126.34. Kim HA, Lee E, Lee SK, Park YB, Lee YN, Kang HJ, et al. 2020; Retention rate and safety of biosimilar CT-P13 in rheumatoid arthritis: data from the Korean College of Rheumatology Biologics registry. BioDrugs. 34:89–98. DOI: 10.1007/s40259-019-00393-y. PMID: 31734899. PMCID: PMC6985057.
Article35. Kim HA, Lee E, Lee SK, Park YB, Lee YN, Kang HJ, et al. 2020; Retention rate and long-term safety of biosimilar CT-P13 in patients with ankylosing spondylitis: data from the Korean College of Rheumatology Biologics registry. Clin Exp Rheumatol. 38:267–74. PMID: 31365335.36. Kim HA, Lee E, Lee SK, Park YB, Shin K. 2020; Retention rate and efficacy of the biosimilar CT-P13 versus reference infliximab in patients with ankylosing spondylitis: a propensity score-matched analysis from the Korean College of Rheumatology Biologics Registry. BioDrugs. 34:529–39. DOI: 10.1007/s40259-020-00432-z. PMID: 32696266. PMCID: PMC7391390.
Article37. Hetland ML. 2005; DANBIO: a nationwide registry of biological therapies in Denmark. Clin Exp Rheumatol. 23(5 Suppl 39):S205–7. PMID: 16273809.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Primary registry of the WHO International Clinical Trial Registry Platform: Clinical Research Information Service (CRIS)
- Cross-platform digital assessment forms for evaluating surgical skills
- Implications of Persistent Pain in Patients With Rheumatoid Arthritis Despite Remission Status: Data From the KOBIO Registry
- A DNA Microarray LIMS System for Integral Genomic Analysis of Multi-Platform Microarrays
- Development of Software Solutions for Stroke: A Personal Experience