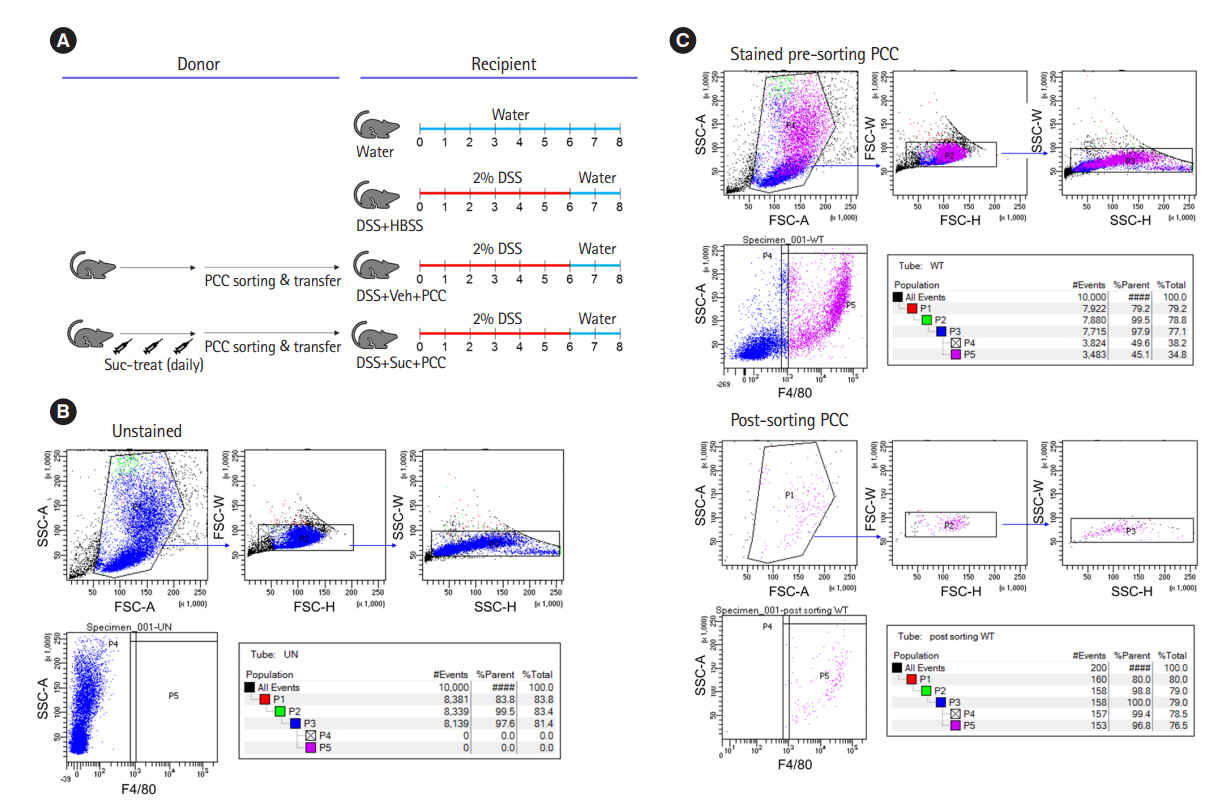
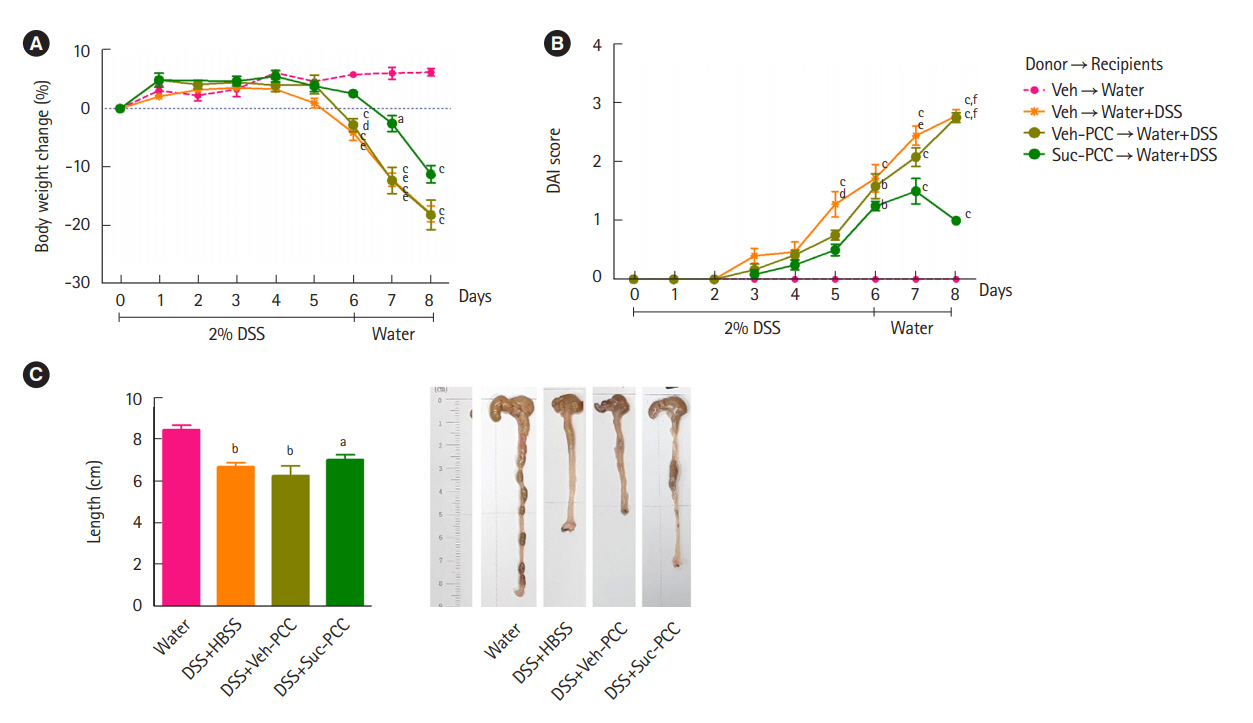
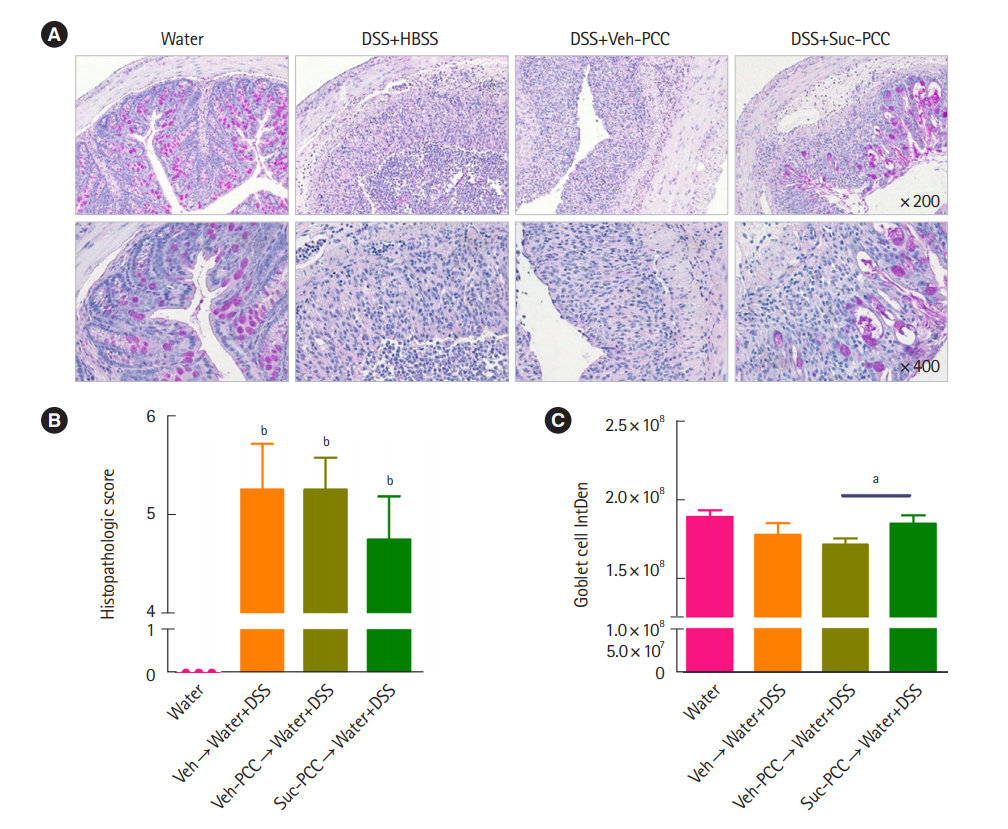
Intest Res.
2021 Jul;19(3):349-353. 10.5217/ir.2020.00075.
Succinate-treated macrophages attenuate dextran sodium sulfate colitis in mice
- Affiliations
-
- 1Department of Internal Medicine and Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea
- 2Brain Korea 21 PLUS Project for Medical Science, Yonsei University College of Medicine, Seoul, Korea
- 3Severance Biomedical Science Institute, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2518691
- DOI: http://doi.org/10.5217/ir.2020.00075
Abstract
- Background/Aims
The safety and effectiveness of adalimumab was demonstrated in a phase 3 trial in Japanese patients with intestinal Behçet’s disease. The aim of this study was to evaluate the long-term safety and effectiveness of adalimumab in Japanese patients with intestinal Behçet’s disease.
Figure
Cited by 2 articles
-
Anti-inflammatory properties of
Escherichia coli Nissle 1917 in a murine colitis model
Jihye Park, Da Hye Kim, Soochan Kim, Hyun Woo Ma, I Seul Park, Mijeong Son, Ji Hyung Kim, Yoojin Shin, Seung Won Kim, Jae Hee Cheon
Intest Res. 2021;19(4):478-481. doi: 10.5217/ir.2021.00121.Anti-inflammatory properties of butyrate-producing atypical
Escherichia coli in a murine colitis model
Ji Hyung Kim, Jee In Yoo, Hyun Woo Ma, I Seul Park, Mijeong Son, Yoojin Shin, Ki Beom Kim, Seung Won Kim, Si Jae Park, Jihye Park
Intest Res. 2023;21(2):266-269. doi: 10.5217/ir.2022.00112.
Reference
-
1. Lloyd-Price J, Arze C, Ananthakrishnan AN, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019; 569:655–662.
Article2. Diskin C, Pålsson-McDermott EM. Metabolic modulation in macrophage effector function. Front Immunol. 2018; 9:270.
Article3. Viola A, Munari F, Sánchez-Rodríguez R, Scolaro T, Castegna A. The metabolic signature of macrophage responses. Front Immunol. 2019; 10:1462.
Article4. Mizoguchi E, Low D, Ezaki Y, Okada T. Recent updates on the basic mechanisms and pathogenesis of inflammatory bowel diseases in experimental animal models. Intest Res. 2020; 18:151–167.
Article5. Macias-Ceja DC, Ortiz-Masiá D, Salvador P, et al. Succinate receptor mediates intestinal inflammation and fibrosis. Mucosal Immunol. 2019; 12:178–187.
Article6. Wang W, Li X, Zheng D, et al. Dynamic changes of peritoneal macrophages and subpopulations during ulcerative colitis to metastasis of colorectal carcinoma in a mouse model. Inflamm Res. 2013; 62:669–680.
Article7. Seo DH, Che X, Kwak MS, et al. Interleukin-33 regulates intestinal inflammation by modulating macrophages in inflammatory bowel disease. Sci Rep. 2017; 7:851.
Article8. Connors J, Dawe N, Van Limbergen J. The role of succinate in the regulation of intestinal inflammation. Nutrients. 2018; 11:25.
Article9. Wu JY, Huang TW, Hsieh YT, et al. Cancer-derived succinate promotes macrophage polarization and cancer metastasis via succinate receptor. Mol Cell. 2020; 77:213–227.
Article10. Keiran N, Ceperuelo-Mallafré V, Calvo E, et al. SUCNR1 controls an anti-inflammatory program in macrophages to regulate the metabolic response to obesity. Nat Immunol. 2019; 20:581–592.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Th17 Responses Are Not Induced in Dextran Sodium Sulfate Model of Acute Colitis
- Histological Study of Experimental Colitis Induced by Dextran Sulfate Sodium
- Anti-inflammatory effects of mulberry twig extracts on dextran sulfate sodium-induced colitis mouse model
- Fine Structure of Goblet Cell Regeneration on Experimental Colitis Induced by Dextran Sulfate Sodium
- Long-Term Effects of Bone Marrow-Derived Mesenchymal Stem Cells in Dextran Sulfate Sodium-Induced Murine Chronic Colitis