Crosstalk Between Mucosal Inflammation and Bone Metabolism in Chronic Rhinosinusitis
- Affiliations
-
- 1Obstructive Upper airway Research (OUaR) Laboratory, Department of Pharmacology, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Biomedical Sciences, Seoul National University Graduate School, Seoul, Korea
- 3Ischemic/Hypoxic Disease Institute, Seoul National University College of Medicine, Seoul, Korea
- 4Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 5Department of Otorhinolaryngology-Head and Neck Surgery, Seoul National University Hospital, Seoul, Korea
- KMID: 2512880
- DOI: http://doi.org/10.21053/ceo.2020.00416
Abstract
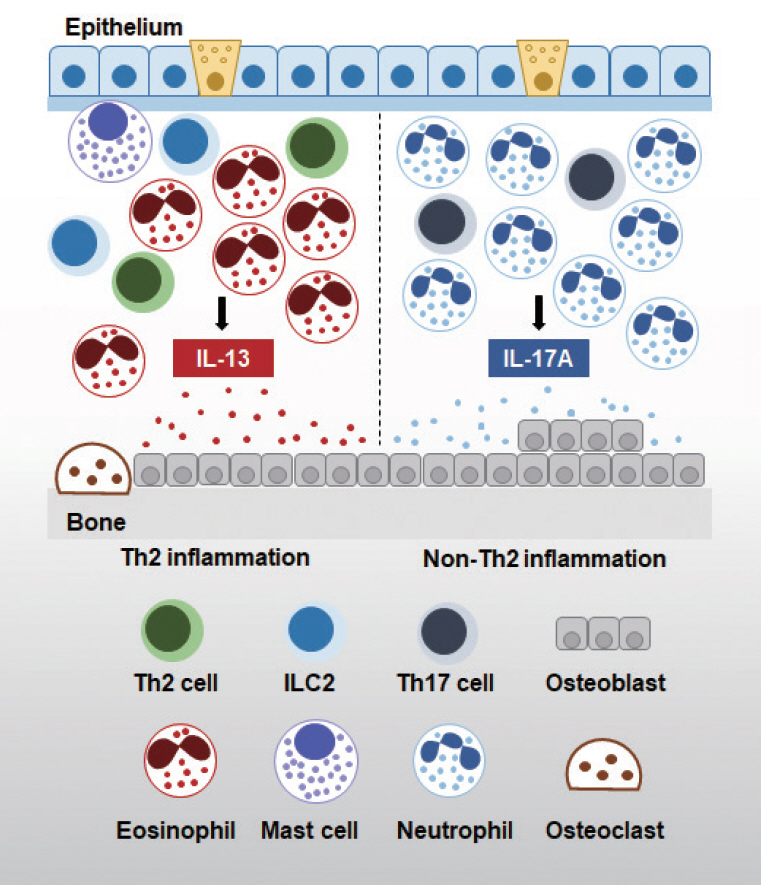
- Chronic rhinosinusitis (CRS) is a multifactorial and highly heterogeneous upper airway disease that affects approximately 12% of the general population. There is increasing evidence supporting the impact of osteitis on the pathophysiology of CRS. Osteitis is frequently observed in patients with CRS, and is associated with severe sinonasal inflammation and recalcitrant cases. The overlying inflammatory sinonasal mucosa plays a critical role in the initiation of osteitis; however, the underlying molecular mechanisms and functional significance remain unclear. Increasingly many studies have suggested that immune cells play a crucial role in the bone remodeling process in CRS. The purpose of this review is to summarize the current state of knowledge regarding the specific role of sinonasal inflammation in bone remodeling in CRS patients.
Keyword
Figure
Cited by 3 articles
-
Effect of Obstructive Sleep Apnea on Immunity in Cases of Chronic Rhinosinusitis With Nasal Polyps
Dong-Kyu Kim, Byeong Chan Lee, Ki Joon Park, Gil Myeong Son
Clin Exp Otorhinolaryngol. 2021;14(4):390-398. doi: 10.21053/ceo.2020.02250.Compositional Alterations of the Nasal Microbiome and
Staphylococcus aureus –Characterized Dysbiosis in the Nasal Mucosa of Patients With Allergic Rhinitis
Hyun Jik Kim, Jong-Hwa Kim, Sun-A Han, Wonyong Kim
Clin Exp Otorhinolaryngol. 2022;15(4):335-345. doi: 10.21053/ceo.2021.01928.A Survey on Biologics for the Treatment of Chronic Rhinosinusitis With Nasal Polyps Among Members of the Korean Rhinologic Society
Hyunkyung Cha, Gwanghui Ryu, Shin Hyuk Yoo, Ji-Hun Mo
J Rhinol. 2023;30(3):155-160. doi: 10.18787/jr.2023.00061.
Reference
-
1. Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012; Mar. 50(1):1–12.
Article2. Hamilos DL. Chronic rhinosinusitis: epidemiology and medical management. J Allergy Clin Immunol. 2011; Oct. 128(4):693–707.
Article3. Schleimer RP. Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Annu Rev Pathol. 2017; Jan. 12:331–57.
Article4. Khalmuratova R, Park JW, Shin HW. Immune cell responses and mucosal barrier disruptions in chronic rhinosinusitis. Immune Netw. 2017; Feb. 17(1):60–7.
Article5. Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013; Jun. 131(6):1479–90.6. Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006; Nov. 61(11):1280–9.
Article7. Brescia G, Zanotti C, Parrino D, Barion U, Marioni G. Nasal polyposis pathophysiology: endotype and phenotype open issues. Am J Otolaryngol. 2018; Jul-Aug. 39(4):441–4.
Article8. Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009; Sep. 124(3):478–84.
Article9. Kim SJ, Lee KH, Kim SW, Cho JS, Park YK, Shin SY. Changes in histological features of nasal polyps in a Korean population over a 17-year period. Otolaryngol Head Neck Surg. 2013; Sep. 149(3):431–7.
Article10. Tikaram A, Prepageran N. Asian nasal polyps: a separate entity. Med J Malaysia. 2013; Dec. 68(6):445–7.11. Cho SW, Kim DW, Kim JW, Lee CH, Rhee CS. Classification of chronic rhinosinusitis according to a nasal polyp and tissue eosinophilia: limitation of current classification system for Asian population. Asia Pac Allergy. 2017; Jul. 7(3):121–30.
Article12. Snidvongs K, Sacks R, Harvey RJ. Osteitis in chronic rhinosinusitis. Curr Allergy Asthma Rep. 2019; Mar. 19(5):24.
Article13. Bhandarkar ND, Sautter NB, Kennedy DW, Smith TL. Osteitis in chronic rhinosinusitis: a review of the literature. Int Forum Allergy Rhinol. 2013; May. 3(5):355–63.
Article14. Snidvongs K, Earls P, Dalgorf D, Sacks R, Pratt E, Harvey RJ. Osteitis is a misnomer: a histopathology study in primary chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014; May. 4(5):390–6.
Article15. Lee JT, Kennedy DW, Palmer JN, Feldman M, Chiu AG. The incidence of concurrent osteitis in patients with chronic rhinosinusitis: a clinicopathological study. Am J Rhinol. 2006; May-Jun. 20(3):278–82.
Article16. Sacks PL, Snidvongs K, Rom D, Earls P, Sacks R, Harvey RJ. The impact of neo-osteogenesis on disease control in chronic rhinosinusitis after primary surgery. Int Forum Allergy Rhinol. 2013; Oct. 3(10):823–7.
Article17. Snidvongs K, McLachlan R, Chin D, Pratt E, Sacks R, Earls P, et al. Osteitic bone: a surrogate marker of eosinophilia in chronic rhinosinusitis. Rhinology. 2012; Sep. 50(3):299–305.
Article18. Georgalas C, Videler W, Freling N, Fokkens W. Global Osteitis Scoring Scale and chronic rhinosinusitis: a marker of revision surgery. Clin Otolaryngol. 2010; Dec. 35(6):455–61.
Article19. Cho SH, Min HJ, Han HX, Paik SS, Kim KR. CT analysis and histopathology of bone remodeling in patients with chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2006; Sep. 135(3):404–8.
Article20. Biedlingmaier JF, Whelan P, Zoarski G, Rothman M. Histopathology and CT analysis of partially resected middle turbinates. Laryngoscope. 1996; Jan. 106(1 Pt 1):102–4.
Article21. Giacchi RJ, Lebowitz RA, Yee HT, Light JP, Jacobs JB. Histopathologic evaluation of the ethmoid bone in chronic sinusitis. Am J Rhinol. 2001; May-Jun. 15(3):193–7.
Article22. Bhandarkar ND, Mace JC, Smith TL. The impact of osteitis on disease severity measures and quality of life outcomes in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2011; Sep-Oct. 1(5):372–8.
Article23. Snidvongs K, McLachlan R, Sacks R, Earls P, Harvey RJ. Correlation of the Kennedy Osteitis Score to clinico-histologic features of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2013; May. 3(5):369–75.
Article24. Meng Y, Lou H, Wang C, Zhang L. Predictive significance of computed tomography in eosinophilic chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2016; Aug. 6(8):812–9.
Article25. Kong IG, Kim DK, Eun KM, Yang SK, Kim M, Oh H, et al. Receptor activator of nuclear factor κB ligand is a biomarker for osteitis of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2020; Mar. 10(3):364–73.
Article26. Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simoes MJ, Cerri PS. Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int. 2015; 2015:421746.
Article27. Caetano-Lopes J, Canhao H, Fonseca JE. Osteoblasts and bone formation. Acta Reumatol Port. 2007; Apr-Jun. 32(2):103–10.28. Metzger CE, Narayanan SA. The role of osteocytes in inflammatory bone loss. Front Endocrinol (Lausanne). 2019; May. 10:285.
Article29. Ishino T, Yajin K, Takeno S, Furukido K, Hirakawa K. Establishment of osteoblast culture from human ethmoidal sinus. Auris Nasus Larynx. 2003; Feb. 30(1):45–51.
Article30. Stevens PR, Tessema B, Brown SM, Parham K, Gronowicz G. Chronic rhinosinusitis osteoblasts differ in cellular properties from normal bone. Int Forum Allergy Rhinol. 2015; Feb. 5(2):124–31.
Article31. Mehta V, Campeau NG, Kita H, Hagan JB. Blood and sputum eosinophil levels in asthma and their relationship to sinus computed tomographic findings. Mayo Clin Proc. 2008; Jun. 83(6):671–8.
Article32. Gunel C, Feldman RE, Bleier BS. Osteitis is associated with P-glycoprotein overexpression in patients with chronic sinusitis without nasal polyps. Am J Rhinol Allergy. 2014; Mar-Apr. 28(2):99–102.33. Miyake MM, Nocera A, Miyake MM. P-glycoprotein and chronic rhinosinusitis. World J Otorhinolaryngol Head Neck Surg. 2018; Aug. 4(3):169–74.
Article34. Cheng YS, Bleier BS. Influence of P-glycoprotein function on chronic rhinosinusitis/nasal polyps pathophysiology. Adv Otorhinolaryngol. 2016; 79:38–47.
Article35. Feldman RE, Lam AC, Sadow PM, Bleier BS. P-glycoprotein is a marker of tissue eosinophilia and radiographic inflammation in chronic rhinosinusitis without nasal polyps. Int Forum Allergy Rhinol. 2013; Aug. 3(8):684–7.
Article36. Bleier BS, Nocera AL, Iqbal H, Hoang JD, Alvarez U, Feldman RE, et al. P-glycoprotein promotes epithelial T helper 2-associated cytokine secretion in chronic sinusitis with nasal polyps. Int Forum Allergy Rhinol. 2014; Jun. 4(6):488–94.
Article37. Bleier BS, Singleton A, Nocera AL, Kocharyan A, Petkova V, Han X. P-glycoprotein regulates Staphylococcus aureus enterotoxin B-stimulated interleukin-5 and thymic stromal lymphopoietin secretion in organotypic mucosal explants. Int Forum Allergy Rhinol. 2016; Feb. 6(2):169–77.38. Onoe Y, Miyaura C, Kaminakayashiki T, Nagai Y, Noguchi K, Chen QR, et al. IL-13 and IL-4 inhibit bone resorption by suppressing cyclooxygenase-2-dependent prostaglandin synthesis in osteoblasts. J Immunol. 1996; Jan. 156(2):758–64.39. Silfversward CJ, Larsson S, Ohlsson C, Frost A, Nilsson O. Reduced cortical bone mass in mice with inactivation of interleukin-4 and interleukin-13. J Orthop Res. 2007; Jun. 25(6):725–31.40. Silfversward CJ, Penno H, Frost A, Nilsson O, Ljunggren O. Expression of markers of activity in cultured human osteoblasts: effects of interleukin-4 and interleukin-13. Scand J Clin Lab Invest. 2010; Sep. 70(5):338–42.41. Tuszynska A, Krzeski A, Postuba M, Paczek L, Wyczalkowska-Tomasik A, Gornicka B, et al. Inflammatory cytokines gene expression in bone tissue from patients with chronic rhinosinusitis: a preliminary study. Rhinology. 2010; Dec. 48(4):415–9.
Article42. Wang M, Ye T, Liang N, Huang Z, Cui S, Li Y, et al. Differing roles for TGF-β/Smad signaling in osteitis in chronic rhinosinusitis with and without nasal polyps. Am J Rhinol Allergy. 2015; Sep-Oct. 29(5):e152–9.
Article43. Gunel C, Bleier BS, Bozkurt G, Eliyatkin N. Microarray analysis of the genes associated with osteitis in chronic rhinosinusitis. Laryngoscope. 2017; Mar. 127(3):E85–90.44. Jin L, Li X. Growth differentiation factor 5 regulation in bone regeneration. Curr Pharm Des. 2013; 19(19):3364–73.
Article45. Sautter NB, Delaney KL, Hausman FA, Trune DR. Tissue remodeling gene expression in a murine model of chronic rhinosinusitis. Laryngoscope. 2012; Apr. 122(4):711–7.
Article46. Wu D, Nocera AL, Mueller SK, Finn K, Libermann TA, Bleier BS. Osteitis is associated with dysregulated pro-osteoblastic activity in patients with nasal polyps. Laryngoscope. 2019; Mar. 129(3):E102–9.
Article47. Oue S, Ramezanpour M, Paramasivan S, Miljkovic D, Cooksley CM, Bassiouni A, et al. Increased IL-13 expression is independently associated with neo-osteogenesis in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2017; Nov. 140(5):1444–8.
Article48. Khalmuratova R, Lee M, Park JW, Shin HW. Evaluation of neo-osteogenesis in eosinophilic chronic rhinosinusitis using a nasal polyp murine model. Allergy Asthma Immunol Res. 2020; Mar. 12(2):306–21.
Article49. Shi N, Zhang J, Chen SY. Runx2, a novel regulator for goblet cell differentiation and asthma development. FASEB J. 2017; Jan. 31(1):412–20.
Article50. Khalmuratova R, Shin HW, Kim DW, Park JW. Interleukin (IL)-13 and IL-17A contribute to neo-osteogenesis in chronic rhinosinusitis by inducing RUNX2. EBioMedicine. 2019; Aug. 46:330–41.
Article51. Komori T. Regulation of bone development and maintenance by Runx2. Front Biosci. 2008; Jan. 13:898–903.
Article52. Jensen ED, Gopalakrishnan R, Westendorf JJ. Regulation of gene expression in osteoblasts. Biofactors. 2010; Jan-Feb. 36(1):25–32.
Article53. Komori T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010; Jan. 339(1):189–95.
Article54. Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008; May. 473(2):139–46.
Article55. Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol. 2014; Oct. 5:511.
Article56. Yeo L, Toellner KM, Salmon M, Filer A, Buckley CD, Raza K, et al. Cytokine mRNA profiling identifies B cells as a major source of RANKL in rheumatoid arthritis. Ann Rheum Dis. 2011; Nov. 70(11):2022–8.
Article57. Ogasawara N, Poposki JA, Klingler AI, Tan BK, Hulse KE, Stevens WW, et al. Role of RANK-L as a potential inducer of ILC2-mediated type 2 inflammation in chronic rhinosinusitis with nasal polyps. Mucosal Immunol. 2020; Jan. 13(1):86–95.
Article58. Huber C, Odermatt A, Hagmann B, Dahinden CA, Fux M. In human basophils, IL-3 selectively induces RANKL expression that is modulated by IgER-dependent and IgER-independent stimuli. Allergy. 2014; Nov. 69(11):1498–505.
Article59. Huang Z, Hajjij A, Li G, Nayak JV, Zhou B, Hwang PH. Clinical predictors of neo-osteogenesis in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015; Apr. 5(4):303–9.
Article60. Karempelis P, Karp E, Rubin N, Hunter R, Dunitz J, Boyer H. Risk factors for neo-osteogenesis in cystic fibrosis and non-cystic fibrosis chronic rhinosinusitis. Int Forum Allergy Rhinol. 2020; Apr. 10(4):505–10.
Article61. Dong D, Yulin Z, Xiao W, Hongyan Z, Jia L, Yan X, et al. Correlation between bacterial biofilms and osteitis in patients with chronic rhinosinusitis. Laryngoscope. 2014; May. 124(5):1071–7.
Article62. Zhao YC, Wormald PJ. Biofilm and osteitis in refractory chronic rhinosinusitis. Otolaryngol Clin North Am. 2017; Feb. 50(1):49–60.
Article63. Braga AA, Valera FC, Faria FM, Rossato M, Murashima AA, Fantucci MZ, et al. An experimental model of eosinophilic chronic rhinosinusitis induced by bacterial toxins in rabbits. Am J Rhinol Allergy. 2019; Nov. 33(6):737–50.
Article64. Cho DY, Mackey C, Van Der Pol WJ, Skinner D, Morrow CD, Schoeb TR, et al. Sinus microanatomy and microbiota in a rabbit model of rhinosinusitis. Front Cell Infect Microbiol. 2018; Jan. 7:540.
Article65. Shin HW. Animal models in CRS and pathophysiologic insights gained: a systematic review. Laryngoscope Investig Otolaryngol. 2016; Aug. 1(5):116–23.
Article66. Perloff JR, Gannon FH, Bolger WE, Montone KT, Orlandi R, Kennedy DW. Bone involvement in sinusitis: an apparent pathway for the spread of disease. Laryngoscope. 2000; Dec. 110(12):2095–9.
Article67. Khalid AN, Hunt J, Perloff JR, Kennedy DW. The role of bone in chronic rhinosinusitis. Laryngoscope. 2002; Nov. 112(11):1951–7.
Article68. Dong Y, Zhou B, Wang X, Huang Z, Wang M, Li Y, et al. Computed tomography and histopathological evaluation of osteitis in rabbit models with rhinosinusitis. Acta Otolaryngol. 2017; May. 137(5):534–40.
Article69. Khalmuratova R, Lee M, Kim DW, Park JW, Shin HW. Induction of nasal polyps using house dust mite and Staphylococcal enterotoxin B in C57BL/6 mice. Allergol Immunopathol (Madr). 2016; Jan-Feb. 44(1):66–75.
Article70. Kim DW, Khalmuratova R, Hur DG, Jeon SY, Kim SW, Shin HW, et al. Staphylococcus aureus enterotoxin B contributes to induction of nasal polypoid lesions in an allergic rhinosinusitis murine model. Am J Rhinol Allergy. 2011; Nov-Dec. 25(6):e255–61.
Article71. Wang S, Zhang H, Xi Z, Huang J, Nie J, Zhou B, et al. Establishment of a mouse model of lipopolysaccharide-induced neutrophilic nasal polyps. Exp Ther Med. 2017; Dec. 14(6):5275–82.
Article72. Lee M, Kim DW, Yoon H, So D, Khalmuratova R, Rhee CS, et al. Sirtuin 1 attenuates nasal polypogenesis by suppressing epithelial-tomesenchymal transition. J Allergy Clin Immunol. 2016; Jan. 137(1):87–98.
Article73. Lee M, Park CG, Huh BK, Kim SN, Lee SH, Khalmuratova R, et al. Sinonasal delivery of resveratrol via mucoadhesive nanostructured microparticles in a nasal polyp mouse model. Sci Rep. 2017; Jan. 7:40249.
Article74. Kim SW, Kim JH, Jung MH, Hur DG, Lee HK, Jeon SY, et al. Periostin may play a protective role in the development of eosinophilic chronic rhinosinusitis with nasal polyps in a mouse model. Laryngoscope. 2013; May. 123(5):1075–81.
Article75. Khalmuratova R, Lee M, Mo JH, Jung Y, Park JW, Shin HW. Wogonin attenuates nasal polyp formation by inducing eosinophil apoptosis through HIF-1α and survivin suppression. Sci Rep. 2018; Apr. 8(1):6201.
Article76. Vandamme TF. Rodent models for human diseases. Eur J Pharmacol. 2015; Jul. 759:84–9.
Article77. Wang Z, Chang L, Huang J, Huang Z, Li X, Chen X, et al. Histological and computed tomographic characteristics of the sinonasal structure of BALB/c mice. Anat Histol Embryol. 2020; Mar. 49(2):222–6.
Article78. Kim SW, Kim DW, Khalmuratova R, Kim JH, Jung MH, Chang DY, et al. Resveratrol prevents development of eosinophilic rhinosinusitis with nasal polyps in a mouse model. Allergy. 2013; Jul. 68(7):862–9.
Article79. Kim Y, Hwang S, Khalmuratova R, Kang S, Lee M, Song Y, et al. α-Helical cell-penetrating peptide-mediated nasal delivery of resveratrol for inhibition of epithelial-to-mesenchymal transition. J Control Release. 2020; Jan. 317:181–94.
Article