J Korean Neurosurg Soc.
2020 Jan;63(1):26-33. 10.3340/jkns.2019.0129.
Glioblastoma Cellular Origin and the Firework Pattern of Cancer Genesis from the Subventricular Zone
- Affiliations
-
- 1Department of Neurosurgery, Brain Tumor Center, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Biochemistry and Molecular Biology, Yonsei University College of Medicine, Seoul, Korea
- 3Medical Research Support Services, Yonsei University College of Medicine, Seoul, Korea
- 4Department of Sculpture, Hongik University, Seoul, Korea
- 5Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Korea
- 6Department of Radiation Oncology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2501690
- DOI: http://doi.org/10.3340/jkns.2019.0129
Abstract
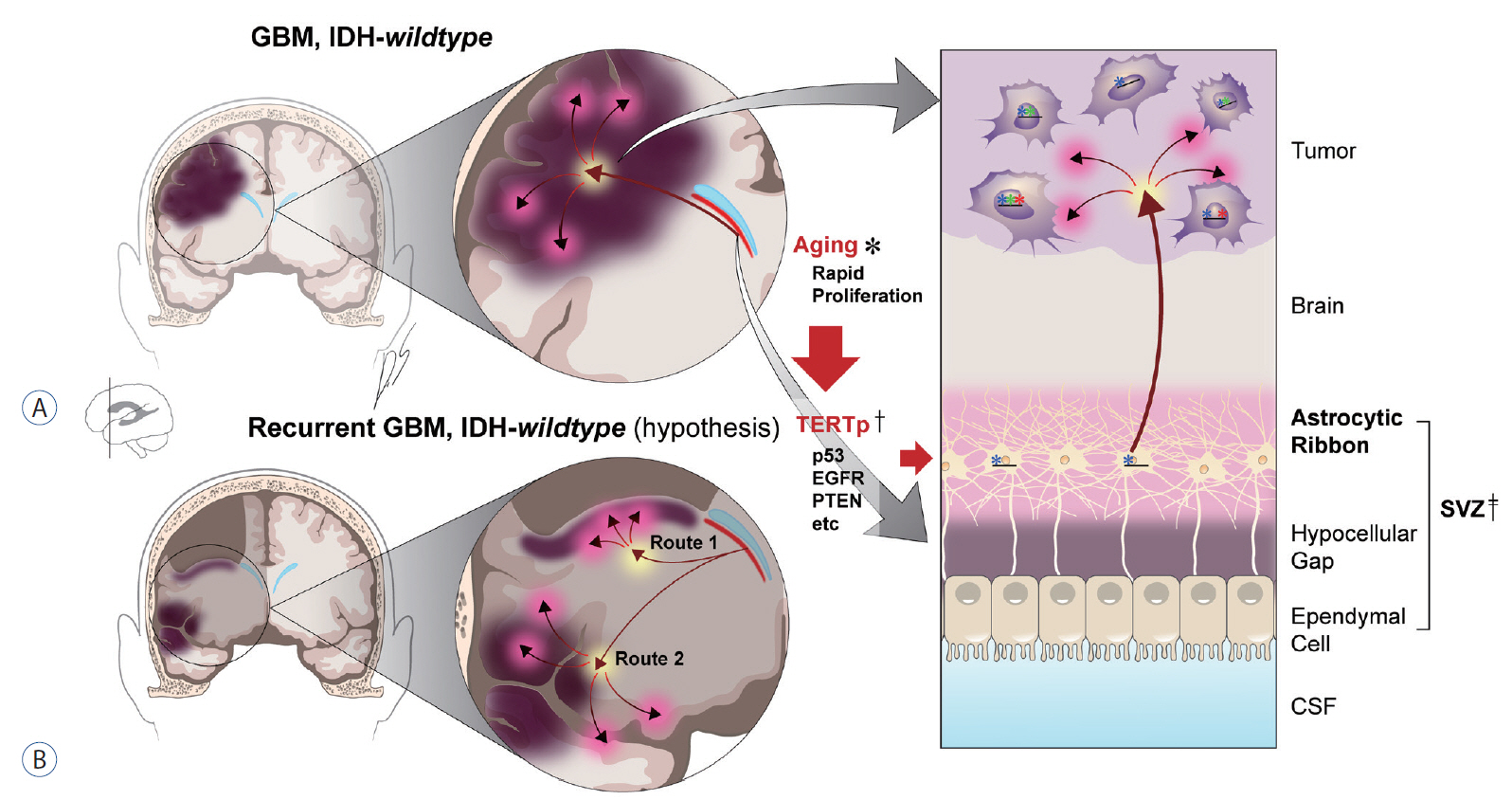
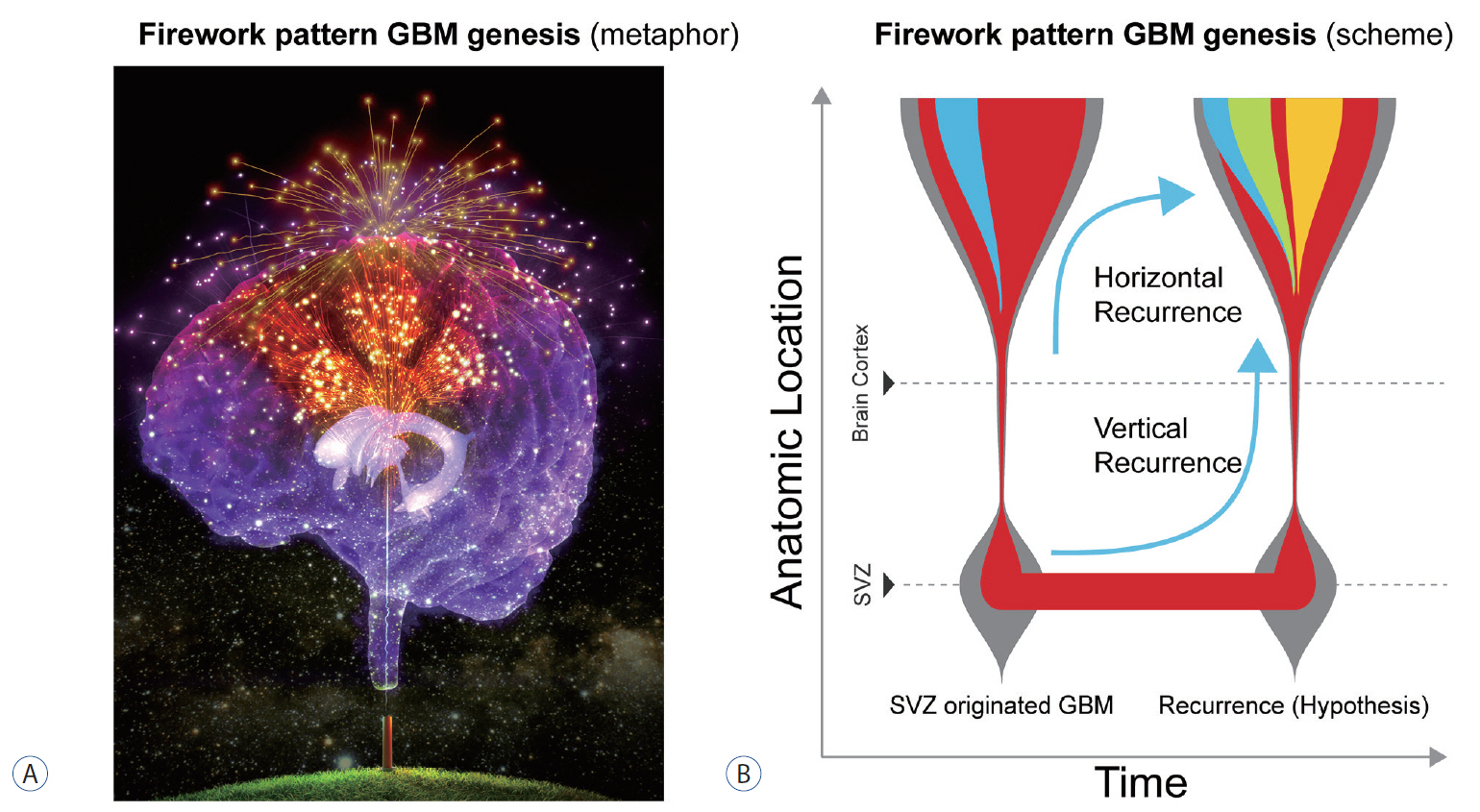
- Glioblastoma (GBM) is a disease without any definite cure. Numerous approaches have been tested in efforts to conquer this brain disease, but patients invariably experience recurrence or develop resistance to treatment. New surgical tools, carefully chosen samples, and experimental methods are enabling discoveries at single-cell resolution. The present article reviews the cell-of-origin of isocitrate dehydrogenase (IDH)-wildtype GBM, beginning with the historical background for focusing on cellular origin and introducing the cancer genesis patterned on firework. The authors also review mutations associated with the senescence process in cells of the subventricular zone (SVZ), and biological validation of somatic mutations in a mouse SVZ model. Understanding GBM would facilitate research on the origin of other cancers and may catalyze the development of new management approaches or treatments against IDH-wildtype GBM.
Keyword
Figure
Reference
-
References
1. Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 15:45–56. 2009.
Article2. Alcantara Llaguno S, Sun D, Pedraza AM, Vera E, Wang Z, Burns DK, et al. Cell-of-origin susceptibility to glioblastoma formation declines with neural lineage restriction. Nat Neurosci. 22:545–555. 2019.
Article3. Alcantara Llaguno SR, Wang Z, Sun D, Chen J, Xu J, Kim E, et al. Adult lineage-restricted cns progenitors specify distinct glioblastoma subtypes. Cancer Cell. 28:429–440. 2015.
Article4. Amirian ES, Zhou R, Wrensch MR, Olson SH, Scheurer ME, Il’yasova D, et al. Approaching a scientific consensus on the association between allergies and glioma risk: a report from the glioma international case-control study. Cancer Epidemiol Biomarkers Prev. 25:282–290. 2016.
Article5. Andersen ZJ, Pedersen M, Weinmayr G, Stafoggia M, Galassi C, Jørgensen JT, et al. Long-term exposure to ambient air pollution and incidence of brain tumor: the european study of cohorts for air pollution effects (escape). Neuro Oncol. 20:420–432. 2018.6. Bae SH, Park MJ, Lee MM, Kim TM, Lee SH, Cho SY, et al. Toxicity profile of temozolomide in the treatment of 300 malignant glioma patients in korea. J Korean Med Sci. 29:980–984. 2014.
Article7. Barthel FP, Wesseling P, Verhaak RGW. Reconstructing the molecular life history of gliomas. Acta Neuropathol. 135:649–670. 2018.
Article8. Braganza MZ, Kitahara CM, Berrington de González A, Inskip PD, Johnson KJ, Rajaraman P. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol. 14:1316–1324. 2012.
Article9. Bräuner EV, Andersen ZJ, Andersen CE, Pedersen C, Gravesen P, Ulbak K, et al. Residential radon and brain tumour incidence in a danish cohort. PLoS One. 8:e74435. 2013.
Article10. Bredel M. Anticancer drug resistance in primary human brain tumors. Brain Res Brain Res Rev. 35:161–204. 2001.
Article11. Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The polymer-brain tumor treatment group. Lancet. 345:1008–1012. 1995.
Article12. Calvert AE, Chalastanis A, Wu Y, Hurley LA, Kouri FM, Bi Y, et al. Cancer-associated idh1 promotes growth and resistance to targeted therapies in the absence of mutation. Cell Rep. 19:1858–1873. 2017.
Article13. Chen J, McKay RM, Parada LF. Malignant glioma: lessons from genomics, mouse models, and stem cells. Cell. 149:36–47. 2012.
Article14. Choi J, Lee JH, Koh I, Shim JK, Park J, Jeon JY, et al. Inhibiting stemness and invasive properties of glioblastoma tumorsphere by combined treatment with temozolomide and a newly designed biguanide (hl156a). Oncotarget. 7:65643–65659. 2016.
Article15. Dipro S, Al-Otaibi F, Alzahrani A, Ulhaq A, Al Shail E. Turcot syndrome: a synchronous clinical presentation of glioblastoma multiforme and adenocarcinoma of the colon. Case Rep Oncol Med. 2012:720273. 2012.
Article16. Fernández ME, Croce S, Boutin C, Cremer H, Raineteau O. Targeted electroporation of defined lateral ventricular walls: a novel and rapid method to study fate specification during postnatal forebrain neurogenesis. Neural Dev. 6:13. 2011.
Article17. Filley AC, Henriquez M, Dey M. Recurrent glioma clinical trial, checkmate-143: the game is not over yet. Oncotarget. 8:91779–91794. 2017.
Article18. Fine HA. The basis for current treatment recommendations for malignant gliomas. J Neurooncol. 20:111–120. 1994.
Article19. Franceschi E, Lamberti G, Paccapelo A, Di Battista M, Genestreti G, Minichillo S, et al. Third-line therapy in recurrent glioblastoma: is it another chance for bevacizumab? J Neurooncol. 139:383–388. 2018.
Article20. Friedmann-Morvinski D, Bushong EA, Ke E, Soda Y, Marumoto T, Singer O, et al. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science. 338:1080–1084. 2012.
Article21. Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 370:699–708. 2014.
Article22. Goffart N, Kroonen J, Rogister B. Glioblastoma-initiating cells: relationship with neural stem cells and the micro-environment. Cancers (Basel). 5:1049–1071. 2013.
Article23. Inskip PD, Sigurdson AJ, Veiga L, Bhatti P, Ronckers C, Rajaraman P, et al. Radiation-related new primary solid cancers in the childhood cancer survivor study: comparative radiation dose response and modification of treatment effects. Int J Radiat Oncol Biol Phys. 94:800–807. 2016.
Article24. INTERPHONE Study Group. Brain tumour risk in relation to mobile telephone use: results of the interphone international case-control study. Int J Epidemiol. 39:675–694. 2010.25. Jeong H, Park J, Shim JK, Lee JE, Kim NH, Kim HS, et al. Combined treatment with 2’-hydroxycinnamaldehyde and temozolomide suppresses glioblastoma tumorspheres by decreasing stemness and invasiveness. J Neurooncol. 143:69–77. 2019.
Article26. Jeong TS, Yee GT. Glioblastoma in a patient with neurofibromatosis type 1: a case report and review of the literature. Brain Tumor Res Treat. 2:36–38. 2014.
Article27. Kang SG, Cheong JH, Huh YM, Kim EH, Kim SH, Chang JH. Potential use of glioblastoma tumorsphere: clinical credentialing. Arch Pharm Res. 38:402–407. 2015.
Article28. Kendall GM, Little MP, Wakeford R, Bunch KJ, Miles JC, Vincent TJ, et al. A record-based case-control study of natural background radiation and the incidence of childhood leukaemia and other cancers in great britain during 1980-2006. Leukemia. 27:3–9. 2012.
Article29. Khalifa J, Tensaouti F, Lusque A, Plas B, Lotterie JA, Benouaich-Amiel A, et al. Subventricular zones: new key targets for glioblastoma treatment. Radiat Oncol. 12:67. 2017.
Article30. Kim EH, Lee JH, Oh Y, Koh I, Shim JK, Park J, et al. Inhibition of glioblastoma tumorspheres by combined treatment with 2-deoxyglucose and metformin. Neuro Oncol. 19:197–207. 2017.
Article31. Kim S, Alexander CM. Tumorsphere assay provides more accurate prediction of in vivo responses to chemotherapeutics. Biotechnol Lett. 36:481–488. 2014.
Article32. Kinnersley B, Mitchell JS, Gousias K, Schramm J, Idbaih A, Labussière M, et al. Quantifying the heritability of glioma using genome-wide complex trait analysis. Sci Rep. 5:17267. 2015.
Article33. Kitange GJ, Carlson BL, Schroeder MA, Grogan PT, Lamont JD, Decker PA, et al. Induction of mgmt expression is associated with temozolomide resistance in glioblastoma xenografts. Neuro Oncol. 11:281–291. 2009.
Article34. Kong BH, Moon JH, Huh YM, Shim JK, Lee JH, Kim EH, et al. Prognostic value of glioma cancer stem cell isolation in survival of primary glioblastoma patients. Stem Cells Int. 2014:838950. 2014.
Article35. Kong BH, Park NR, Shim JK, Kim BK, Shin HJ, Lee JH, et al. Isolation of glioma cancer stem cells in relation to histological grades in glioma specimens. Childs Nerv Syst. 29:217–229. 2013.
Article36. Kong BH, Shin HD, Kim SH, Mok HS, Shim JK, Lee JH, et al. Increased in vivo angiogenic effect of glioma stromal mesenchymal stem-like cells on glioma cancer stem cells from patients with glioblastoma. Int J Oncol. 42:1754–1762. 2013.
Article37. Kratz CP, Achatz MI, Brugières L, Frebourg T, Garber JE, Greer MC, et al. Cancer screening recommendations for individuals with li-fraumeni syndrome. Clin Cancer Res. 23:e38–e45. 2017.
Article38. Kwak J, Shim JK, Kim DS, Lee JH, Choi J, Park J, et al. Isolation and characterization of tumorspheres from a recurrent pineoblastoma patient: feasibility of a patient-derived xenograft. Int J Oncol. 49:569–578. 2016.
Article39. Laks DR. The assessment of glioblastoma tumorspheres reveals molecular determinants of proliferation and therapeutic response. Ann Arbor: University of California;2015. p. 245.40. Lamberti G, Franceschi E, Brandes AA. The burden of oncology promises not kept in glioblastoma. Future Neurol. 13:1–4. 2018.
Article41. Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev. 29:1203–1217. 2015.
Article42. Lee CH, Yu CC, Wang BY, Chang WW. Tumorsphere as an effective in vitro platform for screening anti-cancer stem cell drugs. Oncotarget. 7:1215–1226. 2015.
Article43. Lee JH, Lee JE, Kahng JY, Kim SH, Park JS, Yoon SJ, et al. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature. 560:243–247. 2018.
Article44. Lee SY. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 3:198–210. 2016.
Article45. Moon JH, Kim SH, Shim JK, Roh TH, Sung KS, Lee JH, et al. Histopathological implications of ventricle wall 5-aminolevulinic acid-induced fluorescence in the absence of tumor involvement on magnetic resonance images. Oncol Rep. 36:837–844. 2016.
Article46. Neglia JP, Robison LL, Stovall M, Liu Y, Packer RJ, Hammond S, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 98:1528–1537. 2006.
Article47. Nourallah B, Digpal R, Jena R, Watts C. Irradiating the subventricular zone in glioblastoma patients: is there a case for a clinical trial? Clin Oncol (R Coll Radiol). 29:26–33. 2017.
Article48. Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 16:896–913. 2014.
Article49. Park J, Shim JK, Kang JH, Choi J, Chang JH, Kim SY, et al. Regulation of bioenergetics through dual inhibition of aldehyde dehydrogenase and mitochondrial complex I suppresses glioblastoma tumorspheres. Neuro Oncol. 20:954–965. 2018.
Article50. Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 380:499–505. 2012.
Article51. Ramon Y Cajal Agüeras S. Pío del río-hortega: a visionary in the pathology of central nervous system tumors. Front Neuroanat. 10:13. 2016.52. Roh TH, Kang SG, Moon JH, Sung KS, Park HH, Kim SH, et al. Survival benefit of lobectomy over gross-total resection without lobectomy in cases of glioblastoma in the noneloquent area: a retrospective study. J Neurosurg. 2019; [Epub ahead of print].
Article53. Roh TH, Park HH, Kang SG, Moon JH, Kim EH, Hong CK, et al. Long-term outcomes of concomitant chemoradiotherapy with temozolomide for newly diagnosed glioblastoma patients: a single-center analysis. Medicine (Baltimore). 96:e7422. 2017.54. Ruder AM, Carreón T, Butler MA, Calvert GM, Davis-King KE, Waters MA, et al. Exposure to farm crops, livestock, and farm tasks and risk of glioma: the upper midwest health study. Am J Epidemiol. 169:1479–1491. 2009.
Article55. Sadetzki S, Chetrit A, Freedman L, Stovall M, Modan B, Novikov I. Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiat Res. 163:424–432. 2005.
Article56. Salcman M. Survival in glioblastoma: historical perspective. Neurosurgery. 7:435–439. 1980.57. Sanai N, Tramontin AD, Quiñones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 427:740–744. 2004.
Article58. Shi Y, Lim SK, Liang Q, Iyer SV, Wang HY, Wang Z, et al. Gboxin is an oxidative phosphorylation inhibitor that targets glioblastoma. Nature. 567:341–346. 2019.
Article59. Shibahara I, Sonoda Y, Suzuki H, Mayama A, Kanamori M, Saito R, et al. Glioblastoma in neurofibromatosis 1 patients without idh1, braf v600e, and tert promoter mutations. Brain Tumor Pathol. 35:10–18. 2018.
Article60. Sottoriva A, Kang H, Ma Z, Graham TA, Salomon MP, Zhao J, et al. A big bang model of human colorectal tumor growth. Nat Genet. 47:209–216. 2015.
Article61. Spiteri I, Caravagna G, Cresswell GD, Vatsiou A, Nichol D, Acar A, et al. Evolutionary dynamics of residual disease in human glioblastoma. Ann Oncol. 30:456–463. 2019.
Article62. Stenning SP, Freedman LS, Bleehen NM. An overview of published results from randomized studies of nitrosoureas in primary high grade malignant glioma. Br J Cancer. 56:89–90. 1987.
Article63. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005.
Article64. Sung KS, Shim JK, Lee JH, Kim SH, Park S, Roh TH, et al. Success of tumorsphere isolation from who grade IV gliomas does not correlate with the weight of fresh tumor specimens: an immunohistochemical characterization of tumorsphere differentiation. Cancer Cell Int. 16:75. 2016.
Article65. Tabata H, Yoshinaga S, Nakajima K. Cytoarchitecture of mouse and human subventricular zone in developing cerebral neocortex. Exp Brain Res. 216:161–168. 2012.
Article66. Tseng WL, Hsu HH, Chen Y, Tseng SH. Tumor recurrence in a glioblastoma patient after discontinuation of prolonged temozolomide treatment. Asian J Neurosurg. 12:727–730. 2017.
Article67. Walker MD, Green SB, Byar DP, Alexander E Jr, Batzdorf U, Brooks WH, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 303:1323–1329. 1980.
Article68. Wenger KJ, Wagner M, You SJ, Franz K, Harter PN, Burger MC, et al. Bevacizumab as a last-line treatment for glioblastoma following failure of radiotherapy, temozolomide and lomustine. Oncol Lett. 14:1141–1146. 2017.
Article69. Wernicke AG, Smith AW, Taube S, Mehta MP. Glioblastoma: radiation treatment margins, how small is large enough? Pract Radiat Oncol. 6:298–305. 2016.
Article70. Wilson CB, Gutin P, Boldrey EB, Drafts D, Levin VA, Enot KJ. Single-agent chemotherapy of brain tumors. A five-year review. Arch Neurol. 33:739–744. 1976.71. Zhu H, Acquaviva J, Ramachandran P, Boskovitz A, Woolfenden S, Pfannl R, et al. Oncogenic egfr signaling cooperates with loss of tumor suppressor gene functions in gliomagenesis. Proc Natl Acad Sci U S A. 106:2712–2716. 2009.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Oral Cavity Burn by Firework Explosion
- Mesenchymal Stem-Like Cells Derived from the Ventricle More Effectively Enhance Invasiveness of Glioblastoma Than Those Derived from the Tumor
- Intractable Headache Related to Intraventricular Glioblastoma: A Case Report and Literature Review
- Adult Neurogenesis and Gliogenesis: Possible Mechanisms for Neurorestoration
- Cerebellar Glioblastoma Presenting as a Cerebellar Hemorrhage in a Child